Novel drug for treating eye disease
A drug and functional technology, applied in the field of medicine, can solve the problem of slow recovery of drugs, and achieve the effects of treating myopia, correcting vision, and improving human immunity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1. The parts by weight of each raw material are aspartic acid 0.001, threonine 0.001, serine 0.001, cystine 0.001, valine 0.001, methionine 0.001, isoleucine 0.001, leucine 0.001 , tyrosine 0.001, glutamic acid 0.001, glycine 0.001, alanine 0.001, lysine 0.001, taurine 0.001, carnosine 0.001, nucleotide 0.001, polypeptide 0.001, vitamin B1 0.001, vitamin B6 0.001, vitamin B12 0.001, calcium 0.0001, iodine 0.0001, magnesium 0.0001.
[0030] A preparation method of a novel drug with the function of treating eye diseases, comprising the following steps:
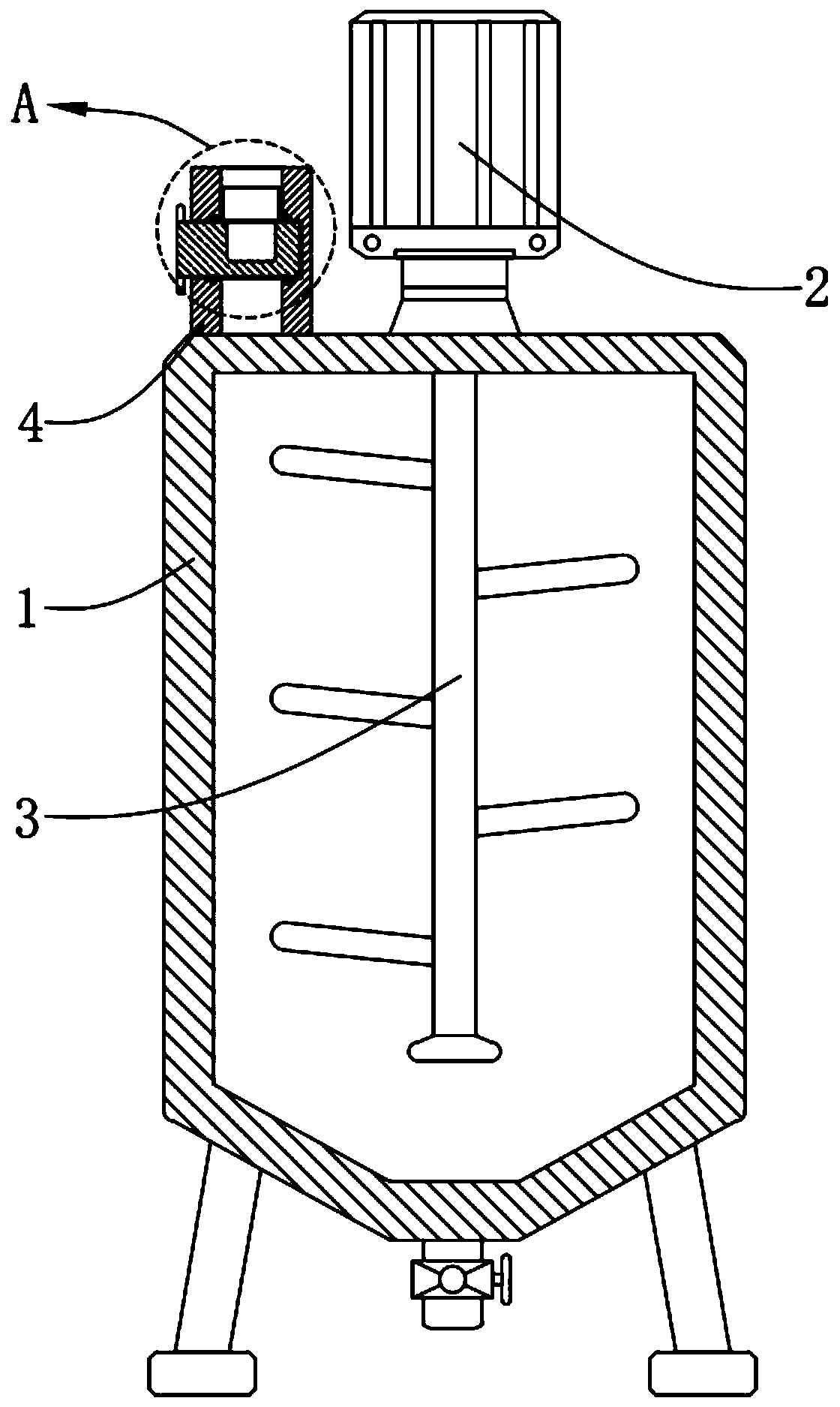
[0031] Each raw material of S1 is weighed according to the ratio of parts by weight, and respectively aspartic acid, threonine, serine, cystine, valine, methionine, isoleucine, leucine, tyrosine, glutamine Acid, glycine, alanine, lysine, taurine, carnosine, nucleotide, polypeptide, vitamin B1, vitamin B6, vitamin B12, calcium, iodine, magnesium are ground to 100 mesh, and the powder after grinding is fully sti...
Embodiment 2
[0056] Embodiment 2, the parts by weight of each raw material are aspartic acid 0.0025, threonine 0.0025, serine 0.0025, cystine 0.0025, valine 0.0025, methionine 0.0025, isoleucine 0.0025, leucine 0.0025 , Tyrosine 0.0025, Glutamic Acid 0.0025, Glycine 0.0025, Alanine 0.0025, Lysine 0.0025, Taurine 0.0025, Carnosine 0.0025, Nucleotide 0.0025, Peptide 0.0025, Vitamin B1 0.0025, Vitamin B6 0.0025, Vitamin B120 .0025, Calcium 0.0002, Iodine 0.0002, Magnesium 0.0002.
Embodiment 3
[0057] Embodiment three, the parts by weight of each raw material are aspartic acid 0.005, threonine 0.005, serine 0.005, cystine 0.005, valine 0.005, methionine 0.005, isoleucine 0.005, leucine 0.005 , tyrosine 0.005, glutamic acid 0.005, glycine 0.005, alanine 0.005, lysine 0.005, taurine 0.005, carnosine 0.005, nucleotide 0.005, polypeptide 0.005, vitamin B1 0.005, vitamin B6 0.005, vitamin B12 0.005, calcium 0.0004, iodine 0.0004, magnesium 0.0004.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com