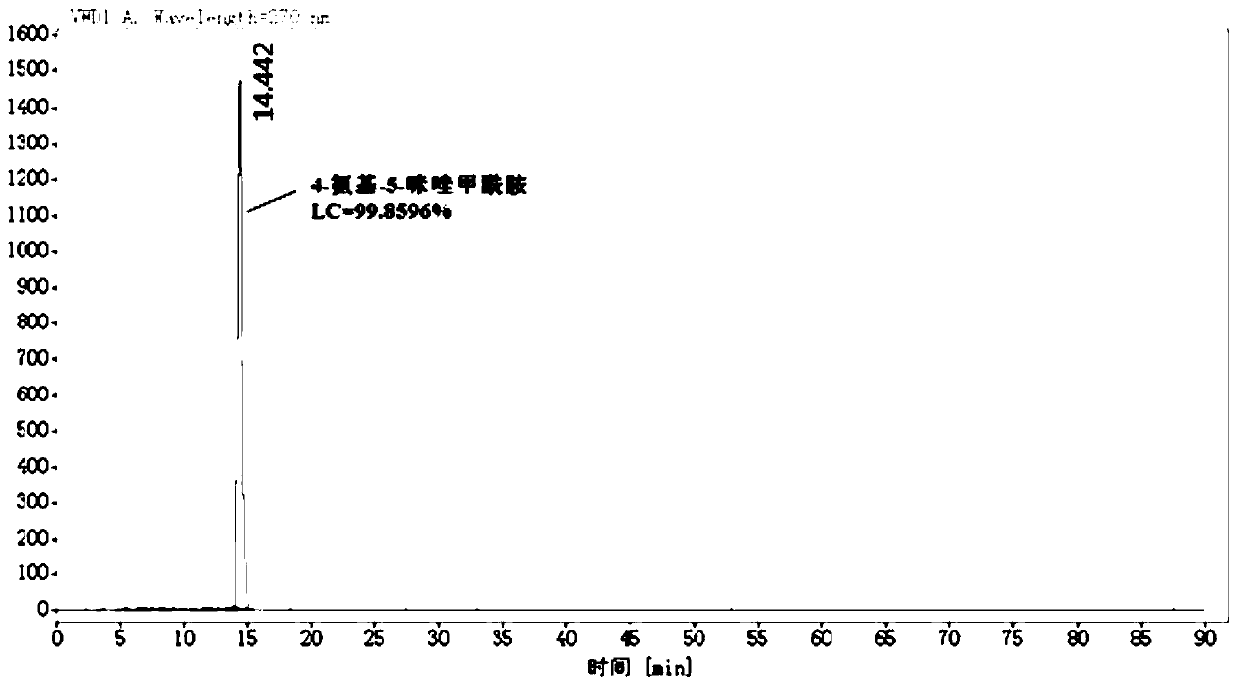
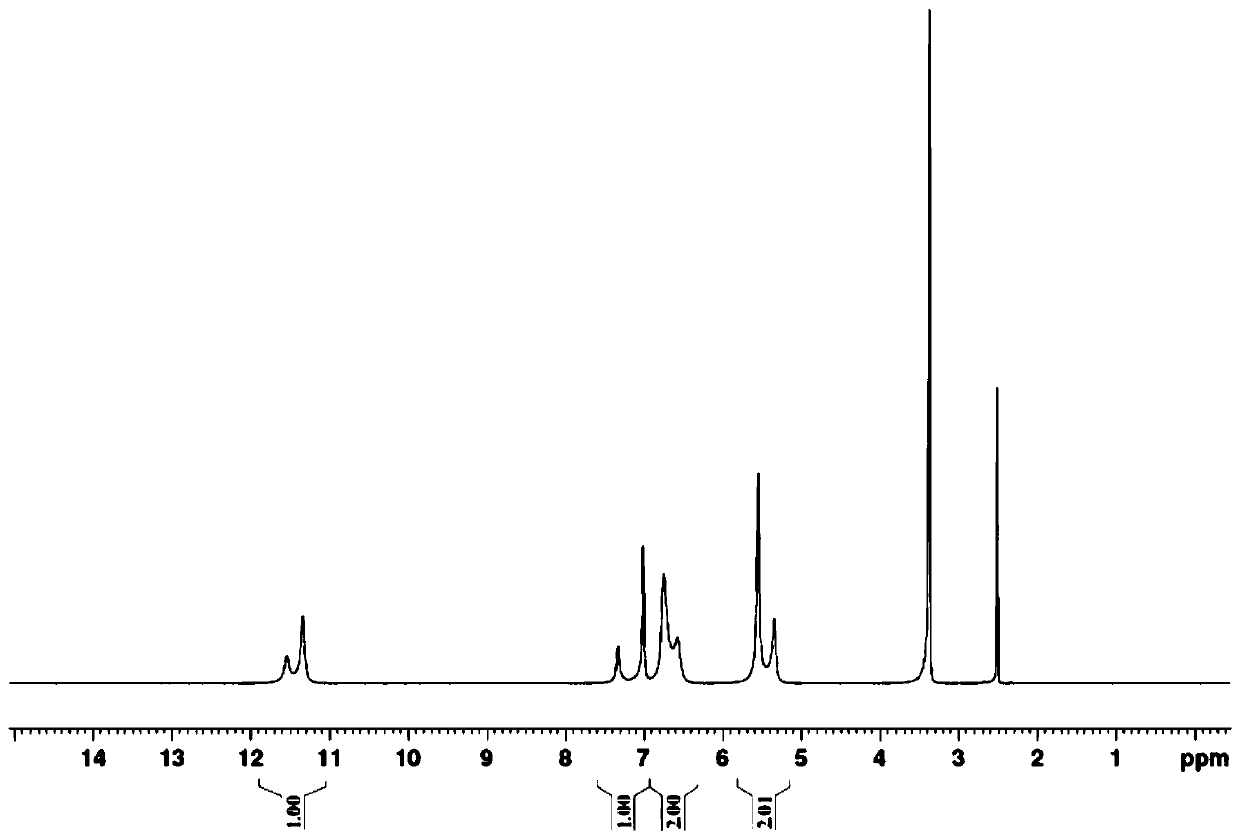
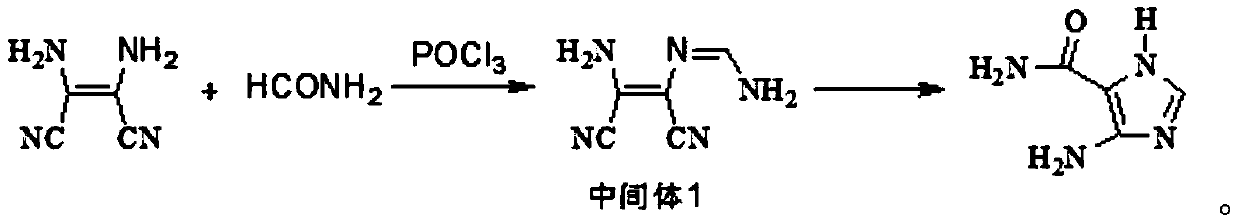
Industrial production method of 4-amino-5-imidazolecarboxamide
A technology of imidazole carboxamide and production method is applied in the field of industrialized production of 4-amino-5-imidazole carboxamide, and can solve the problem that 4-amino-5-imidazole carboxamide cannot meet industrialized production, cannot generate economic benefits, and has no large-scale production. Scale supply and other issues, to achieve the effect of low price, stable quality and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A kind of industrialized production method of 4-amino-5-imidazole carboxamide, specifically comprises the following steps:
[0042] Step 1, synthesis of intermediate 1:
[0043] Under the protection of argon, add THF 1620mL, diaminomaleonitrile 324g, formamide 162g (1.2eq.) to a 5L three-necked flask equipped with a thermometer, dropping funnel and mechanical stirring, and cool down to 5°C in a cryogenic bath , began to add 550.8g (1.2eq.) of phosphorus oxychloride dropwise, and kept warm at 5°C for 2 hours after dropping, and began to take samples every 1 hour. When diaminomaleonitrile LC<0.3%, stop the reaction.
[0044] Post-treatment: Control the temperature at 20°C, add methanol 345.6g dropwise to the reaction solution, after the dropwise addition, control the temperature at 20°C for 2 hours, then slowly pour the system into 4.2L of water to quench, after quenching, keep warm at 20°C and stir for 20min , add 795g of sodium carbonate powder in batches until the pH of...
Embodiment 2
[0050] A kind of industrialized production method of 4-amino-5-imidazole carboxamide, comprises the following steps:
[0051] Step 1, synthesis of intermediate 1:
[0052] Under the protection of argon, add THF 1458mL, diaminomaleonitrile 162g, formamide 101.3g (1.5eq.) successively to a 5L three-neck flask equipped with a thermometer, a dropping funnel and a mechanical stirrer, and the low temperature bath is cooled to 0 ℃, start dropwise adding 344.7g (1.5eq.) of phosphorus oxychloride, keep warm at 35℃ for 2h after dropping, start sampling every 1h, stop the reaction when LC<0.3% of diaminomaleonitrile.
[0053] Post-treatment: Control the temperature at 30°C, add methanol 216g dropwise to the reaction liquid, after the dropwise addition is completed, control the temperature at 30°C for 2 hours, then slowly pour the system into 2.6L of water to quench, after quenching, keep warm at 30°C and stir for 20min, Add 487g of sodium carbonate powder in batches until the system pH=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com