Rhodojaponin III hapten as well as preparation method and application thereof
A technology of halotoxin and hapten, applied in chemical instruments and methods, animal/human protein, serum albumin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation and Identification of Hapten of Aminotoxin III
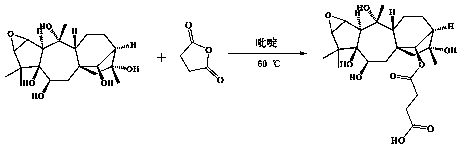
[0056] (1) Dissolve merinotoxin III (20 mg) and succinic anhydride (5.4 mg) in 6.0 mL of anhydrous pyridine, mix the two solutions, and place them in an oil-bath magnetic stirrer for 9 h in the dark (60 o C, 240 rpm), to obtain the reaction product. Synthetic route see figure 1 .
[0057] (2) The reaction system was quenched with water, extracted with ethyl acetate, washed with 0.1M dilute hydrochloric acid, and the organic phase was dried and concentrated.
[0058] (3) Column chromatographic separation to obtain the white target compound haptenin III hapten.
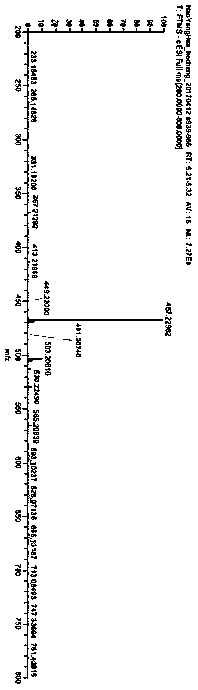
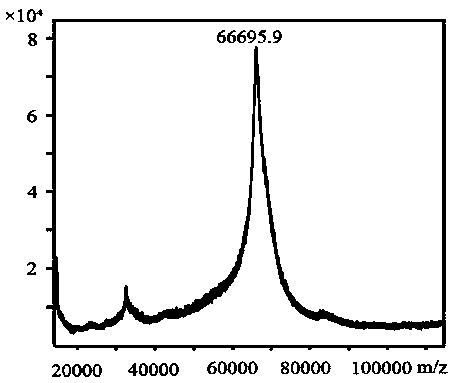
[0059] The hapten toxin III and the purified hapten were identified by liquid phase tandem high-resolution mass spectrometry. According to its synthetic route, if the chemical structure of the target compound is connected with a carbonyl imidazole group, the hapten and the hapten The exact molecular weight is increased by 100 Da compared t...
Embodiment 2
[0060] Example 2 The preparation of the artificial antigen of merinotoxin III
[0061] (1) Weigh the hapten III hapten (10 mg), N-hydroxysuccinimide (4.3 mg) and dicyclohexylcarbodiimide (8.6 mg) prepared in Example 1, respectively, and react with the glass In the bottle, add 1 mL of DMF. The glass bottle containing the reaction solution was placed on a magnetic stirrer, and reacted at room temperature for 5 h at 400 rpm in the dark.
[0062] (2) Dissolve 60 mg of BSA (OVA) in 12 mL of PBS buffer containing 10% (volume percent) DMF to obtain a protein solution.
[0063] (3) Add the liquid phase completed in step (1) dropwise to the protein solution prepared in step (2), place the reaction solution on a magnetic stirrer, and react at 4°C for 5 h.
[0064] (4) The protein activation solution in step (3) was transferred to a dialysis bag with a molecular weight cut-off of 7 KDa, and then the dialysis bag was placed in PBS buffer and dialyzed at 4 °C for 3 days (the medium was c...
Embodiment 3
[0068] Example 3 Preparation and Identification of Polyclonal Antibody to Aminotoxin III
[0069] 1. Preparation of Polyclonal Antibody to Aminotoxin III
[0070] The aminotoxin III-BSA prepared in Example 2 was used as an immunogen to immunize mice, and the aminotoxin III-OVA was used as a coating source to detect the mouse antiserum. The concentration of the complete antigen was determined by the Bradford method, and the concentrations of the immunogen and the coating agent were both 4.5 mg / mL.
[0071] For the first immunization, the immunogen was diluted to 1 mg / mL (diluted with 0.01 M PBS, pH 7.4), and the diluted immunogen was mixed with Freund's complete adjuvant in equal volumes and fully emulsified. Spot inoculate 6-8 week old Balb / c mice (5 mice were immunized), the dose of immunogen was 100 μg / mouse, and the injection dose was 0.2 mL / mouse. Immunization was boosted every 4 weeks, and the immunogen was emulsified with an equal volume of Freund's incomplete adjuvant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Dilution degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com