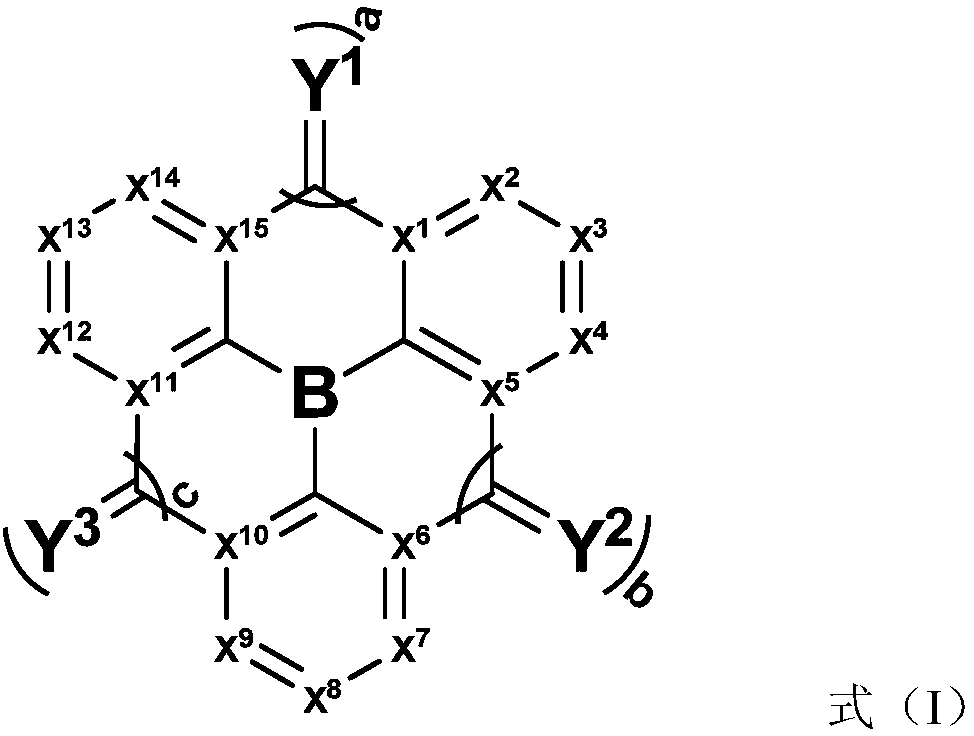
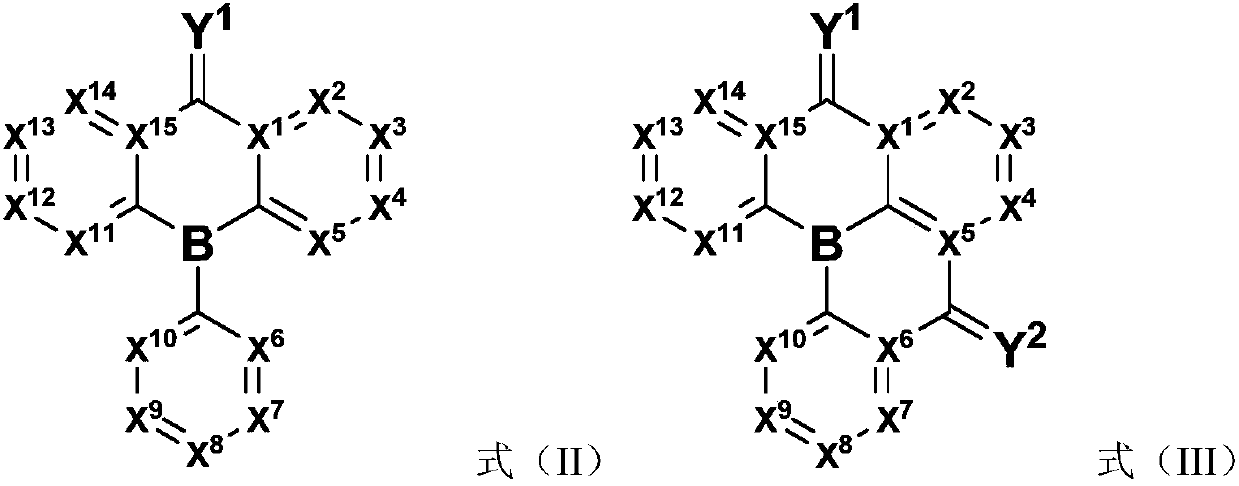
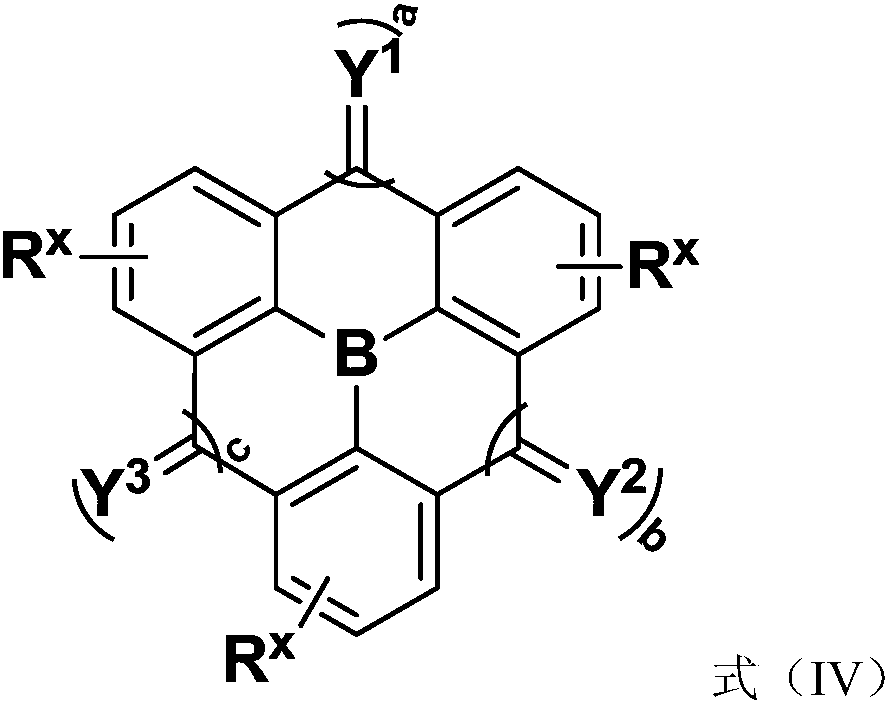
Compound, application thereof, and organic light-emitting device comprising the compound
A compound and an independent technology, applied in the field of organic electroluminescent devices, can solve the problems that the performance needs to be further improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0128] Synthesis of compound M1:
[0129]
[0130] (1) Synthesis of intermediate M1-1
[0131] Add 3.1 g (20 mmol) of bromobenzene and 50 mL of dry toluene into a 250 mL two-necked flask, replace with nitrogen three times and then add to an ice-water bath. Maintaining the ice-water bath, 15 mL (1.6 M, 24 mmol) n-BuLi was added dropwise, and after the addition was completed, it was slowly warmed to room temperature and stirred for 6 h. Weighed 5.4 g (20 mmol) of compound 1 in the glove box, dissolved it in 50 mL of dry toluene solution and added it to the reaction system of the previous step, and continued stirring at room temperature for 20 h. After the reaction was completed, 50 mL of saturated ammonium chloride solution was added to the reaction system to quench, and then extracted with dichloromethane / water. The organic phase was dried and concentrated and then subjected to column chromatography with dichloromethane:petroleum ether=1:10 as the eluent to obtain 4.5 g of...
preparation example 2
[0143] The difference from Preparation Example 1 is that 9,9-dimethylacridine was replaced by 9,9-diphenylacridine in an equivalent amount to obtain 1.0 g of product M2 with a yield of 51.8%.
[0144] The molecular mass determined by mass spectrometry is: 599.35 (calculated value: 599.24); theoretical element content (%) C 44 h 30 BNO: C, 88.15; H, 5.04; B, 1.80; N, 2.34; O, 2.67. Measured element content (%): C, 88.11; H, 5.05; N, 2.29.
[0145] The above analysis results indicated that the obtained product was the expected product.
preparation example 3
[0147] The difference from Preparation Example 1 is that bromobenzene is replaced by 2-bromo-1,3,5-trimethylbenzene of the same amount to obtain 1.2g of product M5 with a yield of 58.6%.
[0148] The molecular mass determined by mass spectrometry is: 517.30 (calculated value: 517.26); theoretical element content (%) C 37 h 32 BNO: C, 85.88; H, 6.23; B, 2.09; N, 2.71; O, 3.09. Measured element content (%): C, 85.89; H, 6.25; N, 2.69.
[0149] The above analysis results indicated that the obtained product was the expected product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com