Method for improving conversion rate in production of iminodisuccinate
A technology of iminodisuccinate and conversion rate, applied in chemical instruments and methods, carboxylate preparation, carboxylate preparation and other directions, can solve problems such as unsatisfactory results and unsatisfactory results, and achieve high conversion rate , the effect of prolonging the reaction time and low content of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 360ml of water in a 1L four-neck flask, start stirring, then add 147g of maleic anhydride, and make maleic anhydride and water carry out hydrolysis reaction to generate maleic acid.
[0035] After the hydrolysis of maleic anhydride is complete, slowly add 233g of solid sodium hydroxide in batches to convert maleic acid into maleic acid salt. This process exotherms strongly, and cooling is required to control the temperature not to exceed 80°C to prevent bumping.
[0036] Then add 210 g of aspartic acid in batches and stir to dissolve completely.
[0037] The molar ratio of maleate to aspartate is 1:1.05, the solid content is about 58%, and the pH value of 1% diluent is 11.46 at room temperature.
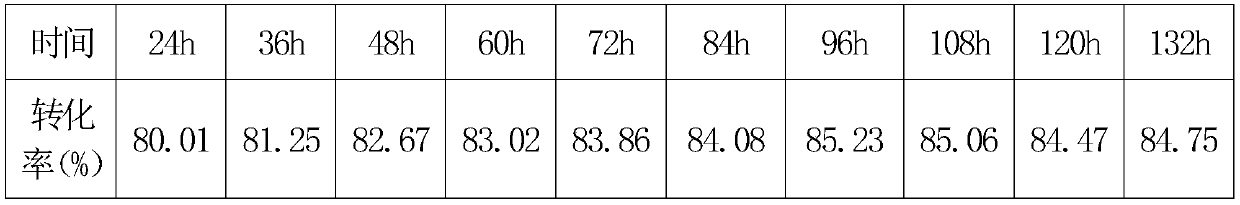
[0038] Next, control the reaction temperature at 90±1°C, and regularly take samples to monitor the conversion rate. The data are as follows:
[0039] The highest conversion rate is at 96 hours, and the sample is diluted to about 40% solid content, which is a transparent...
Embodiment 2
[0041] Add 360ml of water in a 1L four-neck flask, start stirring, then add 147g of maleic anhydride, and make maleic anhydride and water carry out hydrolysis reaction to generate maleic acid.
[0042] After the maleic anhydride is completely hydrolyzed, slowly add 222g of solid sodium hydroxide in batches to convert the maleic acid into maleate. This process is exothermic and requires cooling to control the temperature not to exceed 80°C to prevent bumping.
[0043] Then add 210 g of aspartic acid in batches and stir to dissolve completely.
[0044] The molar ratio of maleate to aspartate is 1:1.05, the solid content is about 57%, and the pH value of 1% diluent is 10.68 at room temperature.
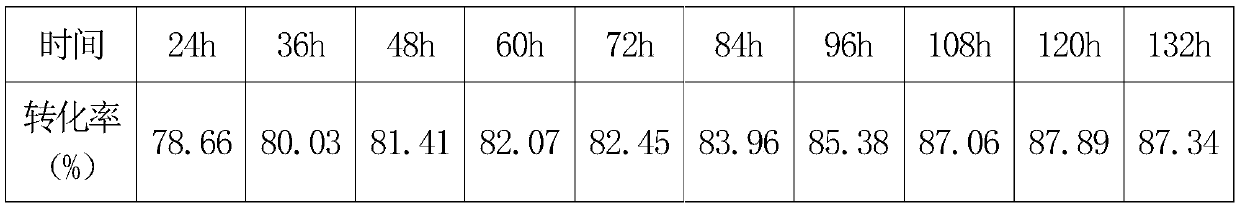
[0045] Next, control the reaction temperature at 90±1°C, and regularly take samples to monitor the conversion rate. The data are as follows:
[0046]
[0047] The highest conversion rate was 85.23% in 96 hours, and the sample was diluted to about 40% solid content, which was a transp...
Embodiment 3
[0050] Add 360ml of water in a 1L four-neck flask, start stirring, then add 147g of maleic anhydride, and make maleic anhydride and water carry out hydrolysis reaction to generate maleic acid.
[0051] After the maleic anhydride is completely hydrolyzed, slowly add 222g of solid sodium hydroxide in batches to convert the maleic acid into maleate. This process is exothermic and requires cooling to control the temperature not to exceed 80°C to prevent bumping.
[0052] Then add 210 g of aspartic acid in batches and stir to dissolve completely.
[0053] The molar ratio of maleate to aspartate is 1:1.05, the solid content is about 57%, and the pH value of 1% diluent is 10.68 at room temperature.
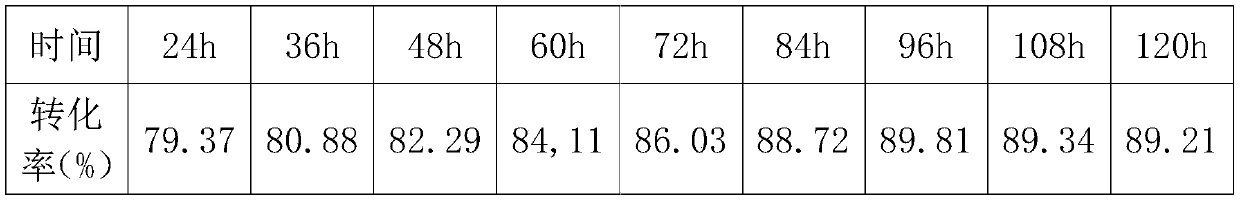
[0054] Next, control the reaction temperature at 85±1°C, and regularly take samples to monitor the conversion rate. The data are as follows:
[0055]
[0056] The highest conversion rate was 87.89% in 120 hours. The sample was diluted to about 40% solid content, which was a transpare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com