Preparation method of alkyl aromatic compound based on alkenyl ether Friedel-Crafts reaction
A technology of aromatic compounds and alkenyl ethers, which is applied in pharmaceutical and chemical intermediates and related chemical fields, can solve the problems of less research on new alkylation reagents and catalytic systems, harsh reaction conditions, complex products, etc., and achieve a wide range of substrate applications , high selectivity, good selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
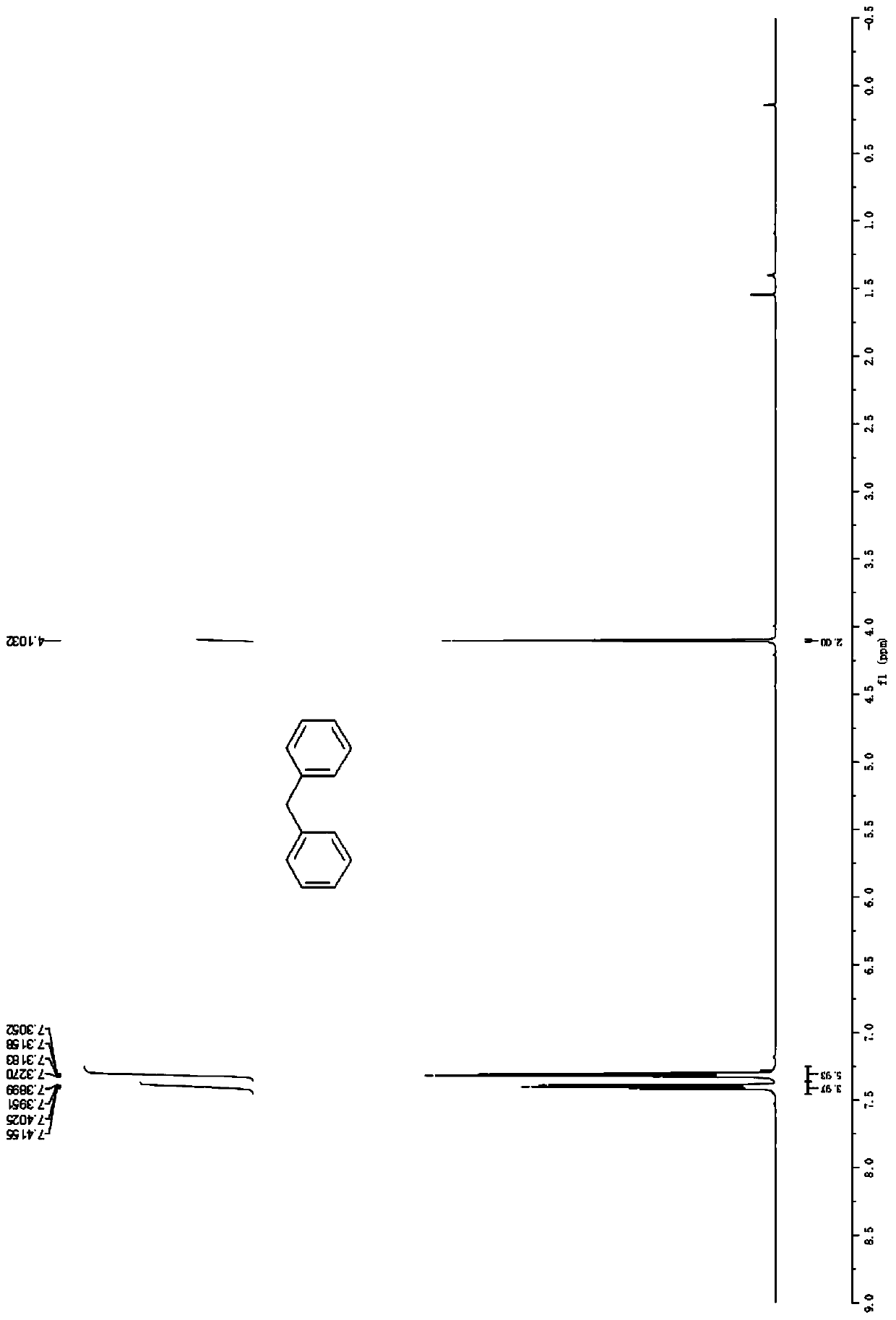
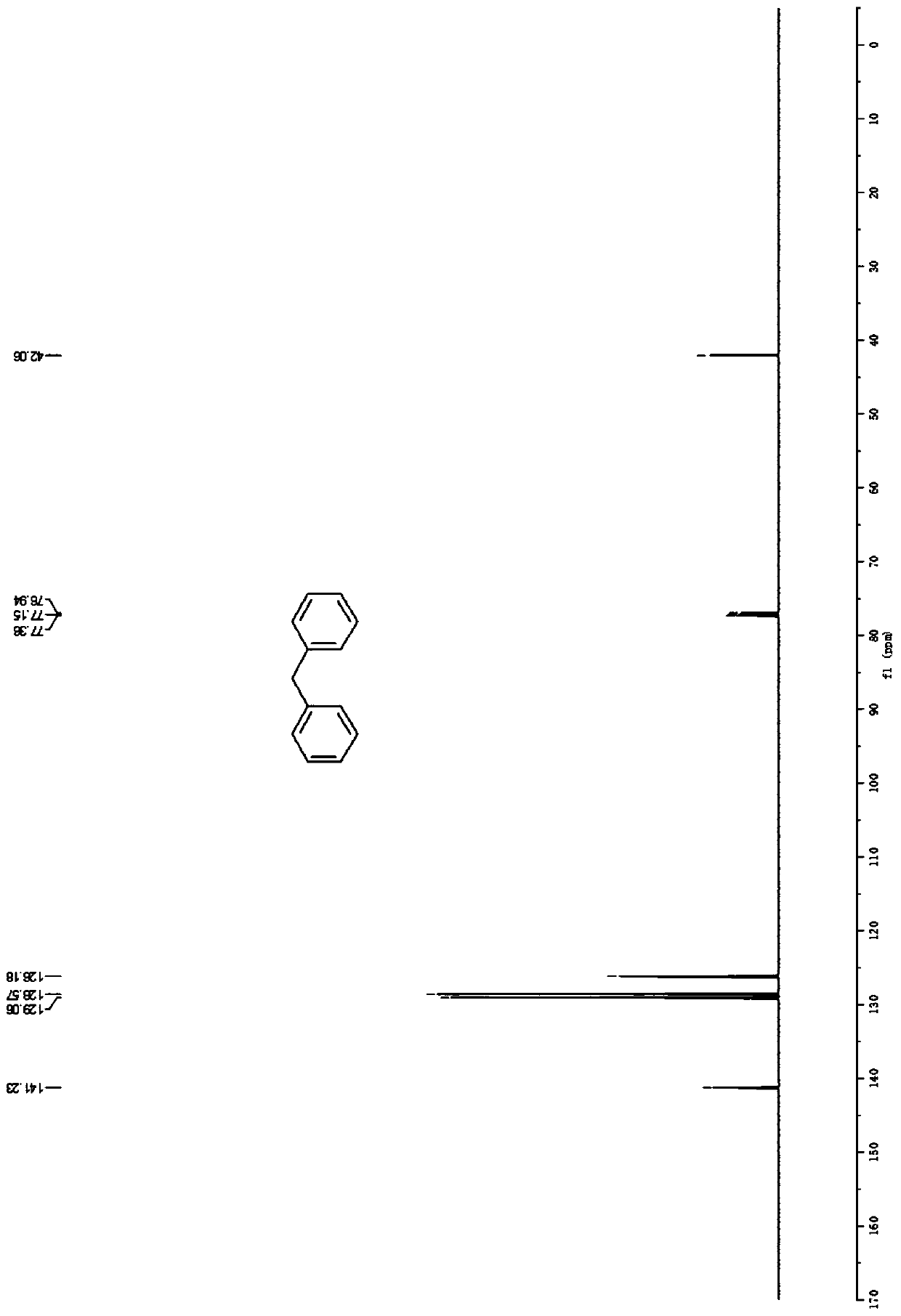
[0036] Embodiment 1: the synthesis of diphenylmethane (3a)
[0037]
[0038] Accurately weigh benzene (78.1mg, 1.0mmol), vinylbenzyl ether (134.2mg, 1.0mmol), zinc chloride (6.8mg, 0.05mmol), and sequentially add to a 50mL Schlenk bottle, add 1,4 - Dioxane (2.0 mL), placed in an oil bath at 60° C. for 16 h. After the reaction was completed, the solvent was distilled off under reduced pressure, and petroleum ether was used as an eluent, and silica gel column chromatography was used for separation, and the yield of diphenylmethane was >99%. 1 H NMR (600MHz, CDCl 3 )δ7.42-7.39(m,4H),7.33-7.31(m,6H),4.10(s,2H); 13 C NMR (151MHz, CDCl 3 ) δ 141.2, 129.1, 128.6, 126.2, 42.1.
Embodiment 2
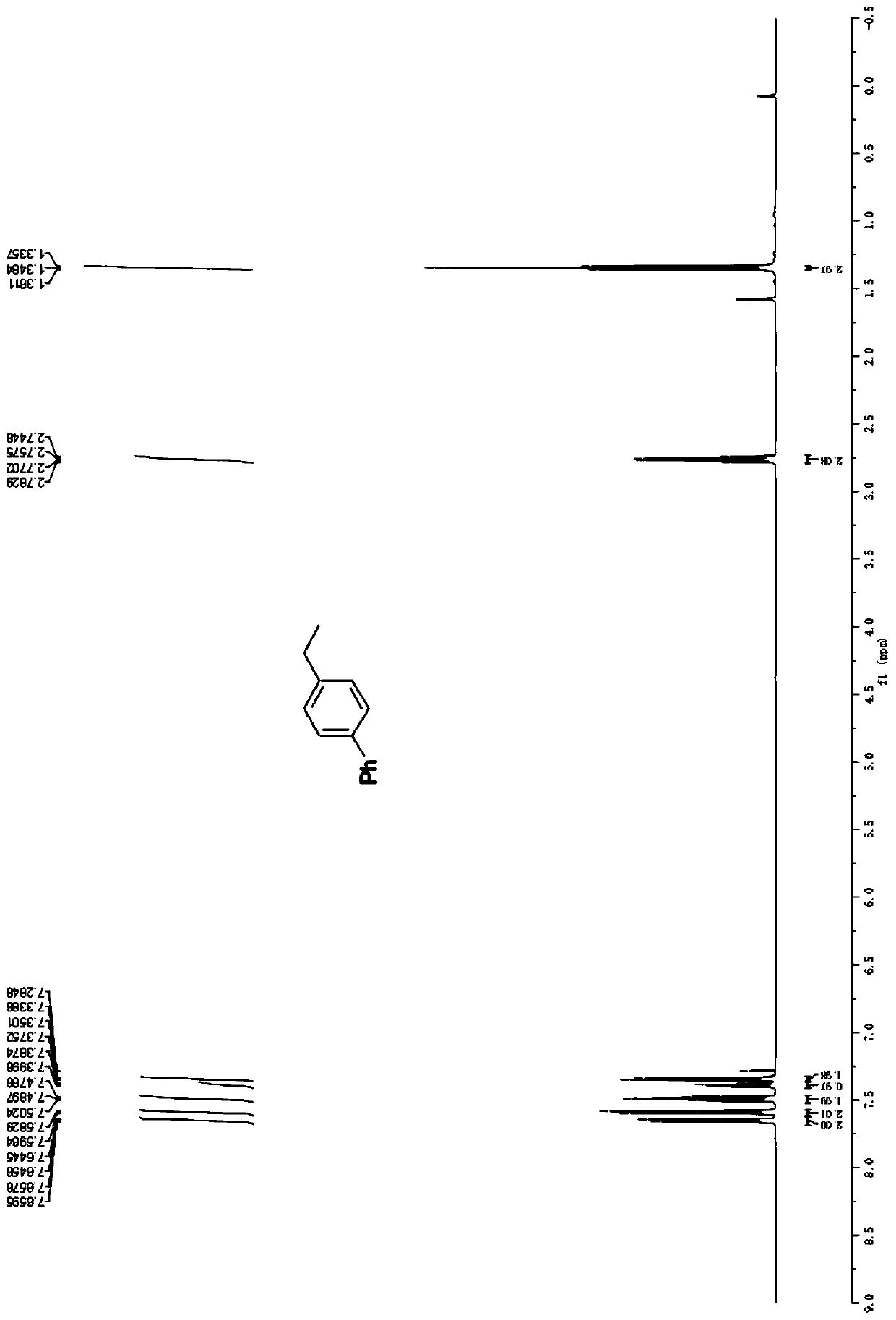
[0039] Example 2: Synthesis of 4-ethyl-1,1'-biphenyl (3b)
[0040]
[0041] Accurately weigh biphenyl (1.54g, 10.0mmol), vinylbenzyl ether (1.44g, 20.0mmol), and p-toluenesulfonic acid (19.2mg, 0.1mmol), and add them to a 100mL Schlenk bottle in turn, add methyl tert-butyl ether (60.0 mL), placed in an oil bath at 90°C for 24 hours. After the reaction was completed, the solvent was distilled off under reduced pressure, and petroleum ether was used as an eluent, and silica gel column chromatography was used for separation. The yield of the alkylated product was 94%. 1 H NMR (600MHz, CDCl 3 )δ7.65(dd, J=8.1,0.9Hz,2H),7.59(d,J=8.1Hz,2H),7.49(t,J=7.7Hz,2H),7.39(t,J=7.4Hz, 1H), 7.34(d, J=8.1Hz, 2H), 2.76(q, J=7.6Hz, 2H), 1.35(t, J=7.6Hz, 3H); 13 C NMR (151MHz, CDCl 3 ) δ 143.4, 141.3, 138.7, 128.8, 128.3, 127.13, 127.06, 127.0, 28.6, 15.6.
Embodiment 3
[0042] Embodiment 3: the synthesis of 2-propylphenol (3c)
[0043]
[0044] Accurately weigh phenol (94.1mg, 1.0mmol), vinyl propyl ether (172.0mg, 2.0mmol), silver acetate (8.4mg, 0.05mmol), and sequentially add to a 25mL Schlenk bottle, add trifluoroethanol ( 5.0 mL), placed in an oil bath at 110°C for 18 hours. After the reaction was completed, the solvent was distilled off under reduced pressure, and petroleum ether / ethyl acetate was used as the eluent, and silica gel column chromatography was used for separation. The yield of the alkylated product was 87%. 1 H NMR (600MHz, CDCl 3 )δ7.17(d,J=7.5Hz,1H),7.13(d,J=7.7,1H),6.96-6.89(m,1H),6.81(dd,J=8.0,0.7Hz,1H),5.11 (s,1H),2.67-2.62(m,2H),1.72-1.67(m,2H),1.03(t,J=7.4Hz,3H); 13 C NMR (151MHz, CDCl 3 ) δ 153.5, 130.3, 128.5, 127.1, 120.8, 115.3, 32.0, 23.0, 14.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com