A New Efficient and Dynamic Synthesis and Purification Technology of N-Methylimidazole
A new technology of methylimidazole and new technology, which is applied in the new field of high-efficiency dynamic N-methylimidazole purification, can solve the problems of complex process flow, high energy consumption of reaction, complex purification, etc., achieve short purification process flow and increase product yield , The effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
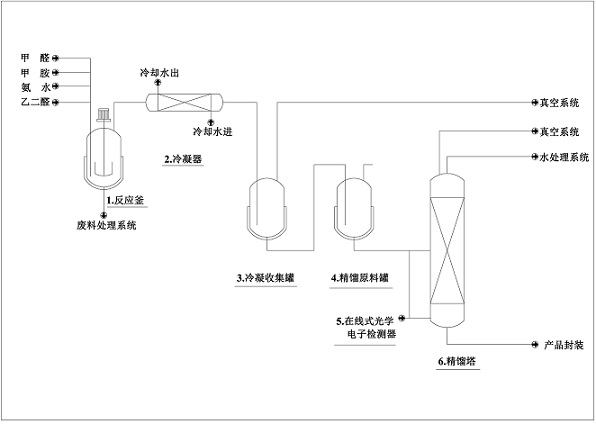
[0023] The high-efficiency dynamic N-methylimidazole purification new technology of the present embodiment, the steps are as follows:
[0024] (1) Add a certain amount of formaldehyde solution into the reaction device, turn on the circulating water bath of the reaction device, keep it at room temperature, and then add 40% methylamine solution and ammonia solution in turn, and the molar ratio of formaldehyde and 40% methylamine solution is 1:2.5 , the molar ratio of formaldehyde and ammonia water is 1:2, and stirring is started at room temperature. After stirring for 10 min, the glyoxal solution was slowly added, and the molar ratio of formaldehyde and glyoxal was 1:1.5. After the glyoxal solution was completely added, the temperature of the circulating water bath was raised to 50°C, and the reaction was carried out at this temperature for 5h;
[0025] (2) After the reaction is completed, the reaction device is connected to the vacuum condensation system, the temperature of th...
Embodiment 2
[0029] The high-efficiency dynamic N-methylimidazole purification new technology of the present embodiment, the steps are as follows:
[0030] (1) Add a certain amount of formaldehyde solution into the reaction device, turn on the circulating water bath of the reaction device, keep it at room temperature, and then add 40% methylamine solution and ammonia solution in turn, and the molar ratio of formaldehyde and 40% methylamine solution is 1:4 , the molar ratio of formaldehyde and ammonia water is 1:3, and stirring is started at room temperature. After stirring for 10 min, the glyoxal solution was slowly added, and the molar ratio of formaldehyde and glyoxal was 1:2. After the glyoxal solution was completely added, the temperature of the circulating water bath was raised to 70°C, and the reaction was carried out at this temperature for 3h;
[0031] (2) After the reaction, the reaction device was connected to the vacuum condensation system, the temperature of the circulating wa...
Embodiment 3
[0035] The high-efficiency dynamic N-methylimidazole purification new technology of the present embodiment, the steps are as follows:
[0036] (1) Add a certain amount of formaldehyde solution into the reaction device, turn on the circulating water bath of the reaction device, keep it at room temperature, and then add 40% methylamine solution and ammonia solution in turn, the molar ratio of formaldehyde and 40% methylamine solution is 1:1 , the molar ratio of formaldehyde and ammonia water is 1:1, and stirring is started at room temperature. After stirring for 10 min, the glyoxal solution was slowly added, and the molar ratio of formaldehyde and glyoxal was 1:1. After the glyoxal solution was completely added, the temperature of the circulating water bath was raised to 60°C, and the reaction was carried out at this temperature for 4h;
[0037] (2) After the reaction is completed, the reaction device is connected to the vacuum condensation system, the temperature of the circul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com