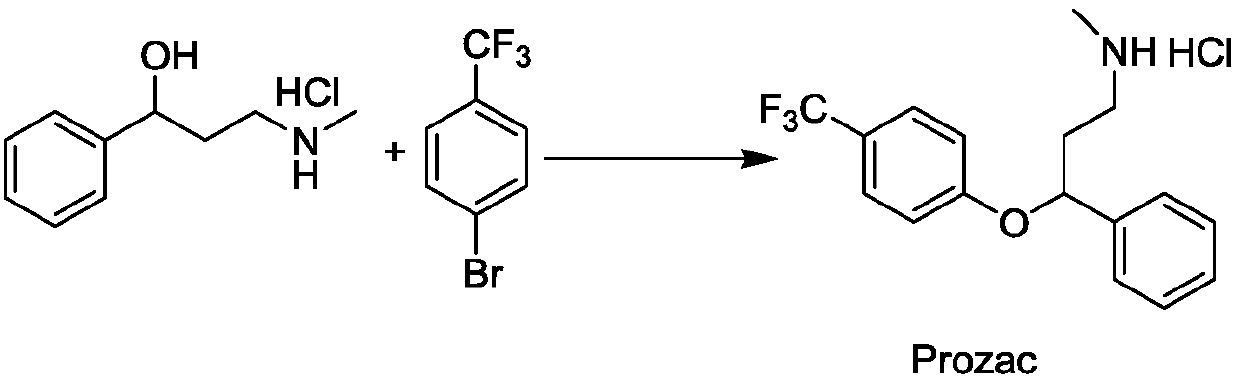
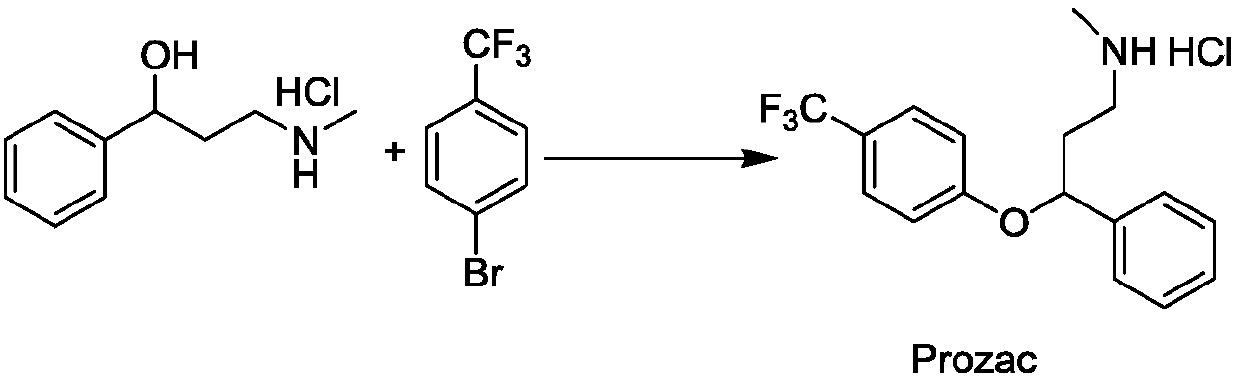
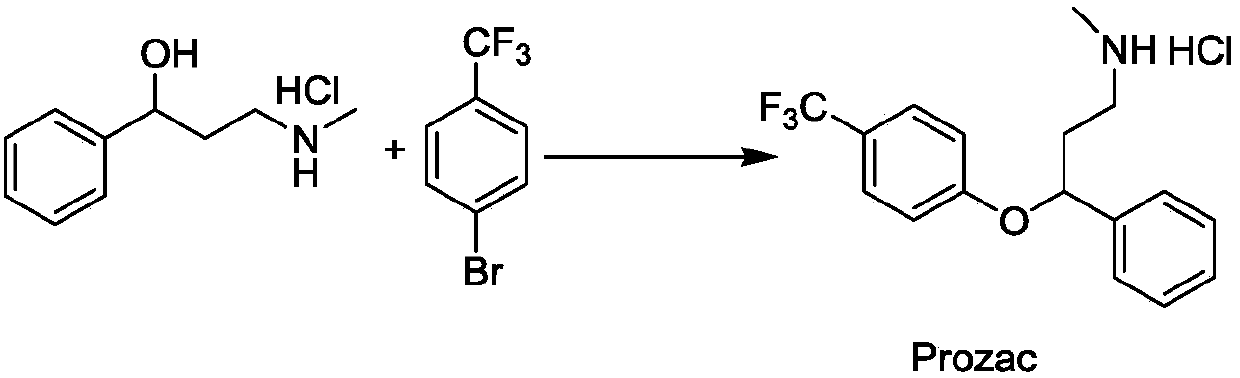
Photochemical catalytic synthesis method of aryl alkyl ether
A technology of aryl alkyl ether and synthesis method, applied in chemical instruments and methods, preparation of aminohydroxy compounds, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Mixed crystal form P25 titanium dioxide, p-bromoacetophenone, bis(triphenylphosphine)nickel dichloride, 4,4'-di-tert-butyl-2,2'-bipyridine, and cesium carbonate at 1:10: Add the molar ratio of 1:1:10 (0.5mmol:5mmol:0.5mmol:0.5mmol:5mmol) into a temperature-controlled transparent reaction bottle filled with methanol and control the temperature at 25°C to make the mixed crystal P25 titanium dioxide in the reaction system The concentration in the reaction system is 40g / L, p-bromoacetophenone, bis(triphenylphosphine) nickel dichloride, 4,4'-di-tert-butyl-2,2'-bipyridyl, cesium carbonate in the reaction system The concentrations are 5mol / L, 0.5mol / L, 0.5mol / L, 5mol / L respectively, the seal is sealed, and the inert gas is introduced, and the pressure of the inert gas in the temperature-controlled transparent reaction bottle is 0.01MPa, and the temperature is controlled at 25 ℃ and stirred for half an hour to make the adsorption of p-bromoacetophenone reach equilibrium, then i...
Embodiment 2
[0068] Anatase titanium dioxide, p-bromobenzonitrile, nickel chloride, 4,4'-dimethoxy-2,2'-bipyridine, ethanol, and potassium carbonate were mixed at 1:20:1:1:20: 20 (0.25mmol: 5mmol: 0.25mmol: 0.25mmol: 5mmol: 5mmol) was added in a temperature-controlled transparent reaction bottle filled with acetonitrile and the temperature was controlled at 25°C, so that the anatase-type titanium dioxide in the reaction system The concentration is 8g / L, and the concentrations of p-bromobenzonitrile, nickel chloride, 4,4'-dimethoxy-2,2'-bipyridine, ethanol, and potassium carbonate in the reaction system are 2mol / L, 0.1mol / L, 0.1mol / L, 2mol / L, 2mol / L), airtightly seal, introduce inert gas, and make the pressure of the inert gas in the temperature-controlled transparent reaction bottle be 0.01MPa, control the temperature at 25°C and stir Make p-bromobenzonitrile adsorption reached equilibrium in half an hour, then irradiate the temperature-controlled transparent reaction bottle with a 300-wat...
Embodiment 3
[0070] The rutile titanium dioxide and o-trifluoromethyl chlorobenzene, tetrakis (triphenylphosphine) nickel, 2,2'-bipyridine, isopropanol, and potassium phosphate were mixed in a ratio of 1:10:10:1:1:10 (0.5 The molar ratio of mmol:5mmol:0.5mmol:0.5mmol:5mmol:5mmol) was added to the temperature-controlled transparent reaction bottle filled with dimethyl sulfoxide and the temperature was controlled at 25°C to make the concentration of rutile titanium dioxide in the reaction system is 16g / L, o-trifluoromethyl chlorobenzene, tetrakis (triphenylphosphine) nickel, bipyridine, isopropanol, and potassium phosphate are respectively 2mol / L, 0.2mol / L, and 0.2mol in the concentration of potassium phosphate / L, 2mol / L, 2mol / L, airtightly seal, introduce an inert gas, and make the pressure of the inert gas in the temperature-controlled transparent reaction bottle be 0.01MPa, control the temperature at 25°C and stir for half an hour to make the o-trifluoromethyl Chlorobenzene adsorption re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com