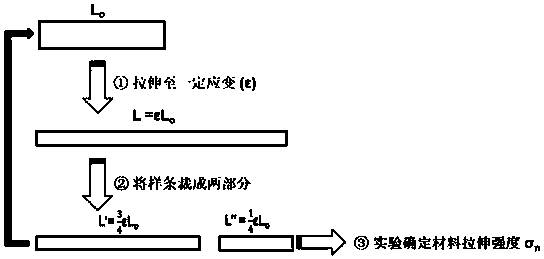
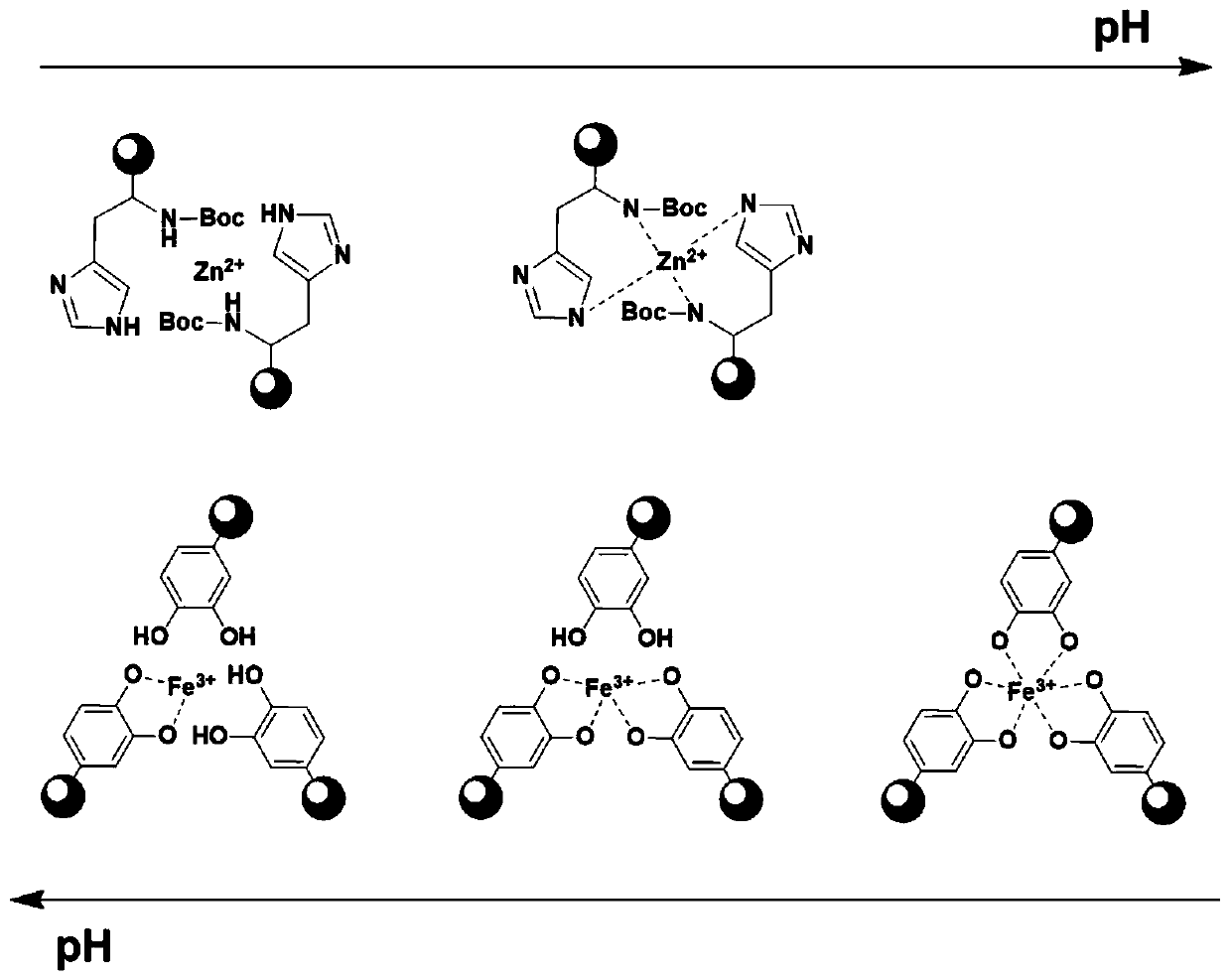
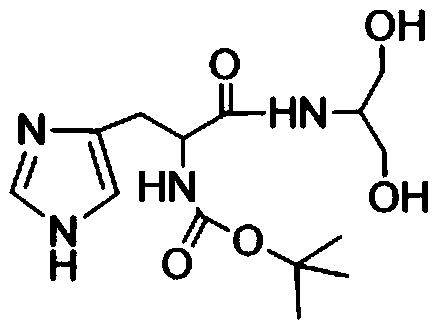
High polymer material capable of realizing self-healing of chemical bond damage under humid and stressed conditions and preparation method of high polymer material
A technology of polymer materials and chemical bonds, which is applied in the field of polymer materials and their preparation, can solve problems such as the inability to maintain long-term stability of mechanical properties, and achieve the effect of strong mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] This example provides a polymer material capable of self-healing of chemical bond damage under moisture and stress conditions, specifically cross-linked polyurethane, and its preparation method is as follows:
[0059] (1) Preparation of polyurethane prepolymer
[0060] Under the protection of high-purity nitrogen, 200g polyethylene glycol (molecular weight is 800g / mol) is added in the three-necked flask, then in the three-necked flask, add 500mL N, N-dimethylformamide, under the mechanical stirring condition above-mentioned Polyethylene glycol is completely dissolved, then 111g of isophorone diisocyanate is added to the above mixed solution through a constant pressure dropping funnel, after mixing evenly, 0.15g of dibutyltin dilaurate is added, and the temperature of the reaction system is adjusted to 40°C. After 12 hours of reaction, a polyurethane prepolymer was prepared.
[0061] (2) Preparation of terminal dihydroxyhistidine monomer
[0062] Dissolve 63g of Boc-L-...
Embodiment 2
[0071] This example provides a polymer material capable of self-healing of chemical bond damage under moisture and stress conditions, specifically cross-linked polyurethane, and its preparation method is as follows:
[0072] (1) Preparation of polyurethane prepolymer
[0073] Under the protection of high-purity nitrogen, add 200g of polytetrahydrofuran diol (molecular weight: 5000g / mol) into the three-necked flask, then add 500mL of dimethylformamide into the three-necked flask, and mix the above polyethylene diol under mechanical stirring Alcohol is completely dissolved, then add 13.2g of dimethyl biphenyl diisocyanate to the above mixed solution through a constant pressure dropping funnel, after mixing evenly, add 0.25g of dibutyltin dilaurate, adjust the temperature of the reaction system to 80°C, react After 12 hours, a polyurethane prepolymer was prepared.
[0074] (2) Preparation of terminal dihydroxyhistidine monomer
[0075] Dissolve 10.1g of Boc-L-histidine and 4.5g...
Embodiment 3
[0084] This example provides a polymer material capable of self-healing of chemical bond damage under moisture and stress conditions, specifically cross-linked polyurethane, and its preparation method is as follows:
[0085] (1) Preparation of polyurethane prepolymer
[0086] Under the protection of high-purity nitrogen, add 200g polypropylene glycol (molecular weight is 2000g / mol) in the three-necked flask, then add 500mL dimethylformamide in the three-necked flask, and dissolve the above-mentioned polyethylene glycol completely under the condition of mechanical stirring , and then add 23.2g of p-phenylene diisocyanate to the above mixed solution through a constant pressure dropping funnel, after mixing evenly, add 0.1g of dibutyltin dilaurate, adjust the temperature of the reaction system to 60°C, and prepare the polyurethane preprepared after 12 hours of reaction Polymer.
[0087] (2) Preparation of terminal dihydroxyhistidine monomer
[0088] Dissolve 25.2g of Boc-L-hist...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com