N/O double-doped metal carbon coated carbide nanoparticle composite material and preparation thereof
A nanoparticle and carbide technology, applied in chemical/physical processes, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as poor stability, high cost, and scarcity of global reserves, and achieve simple preparation processes , the effect of high scientific research value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
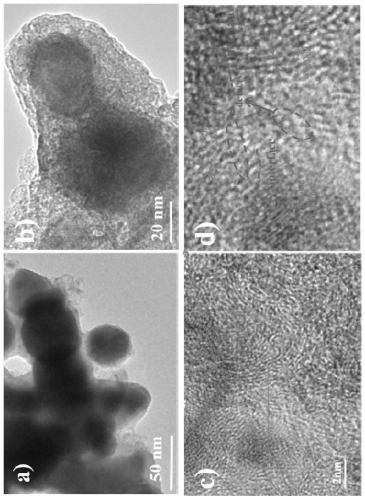
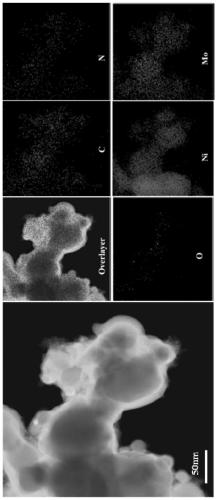
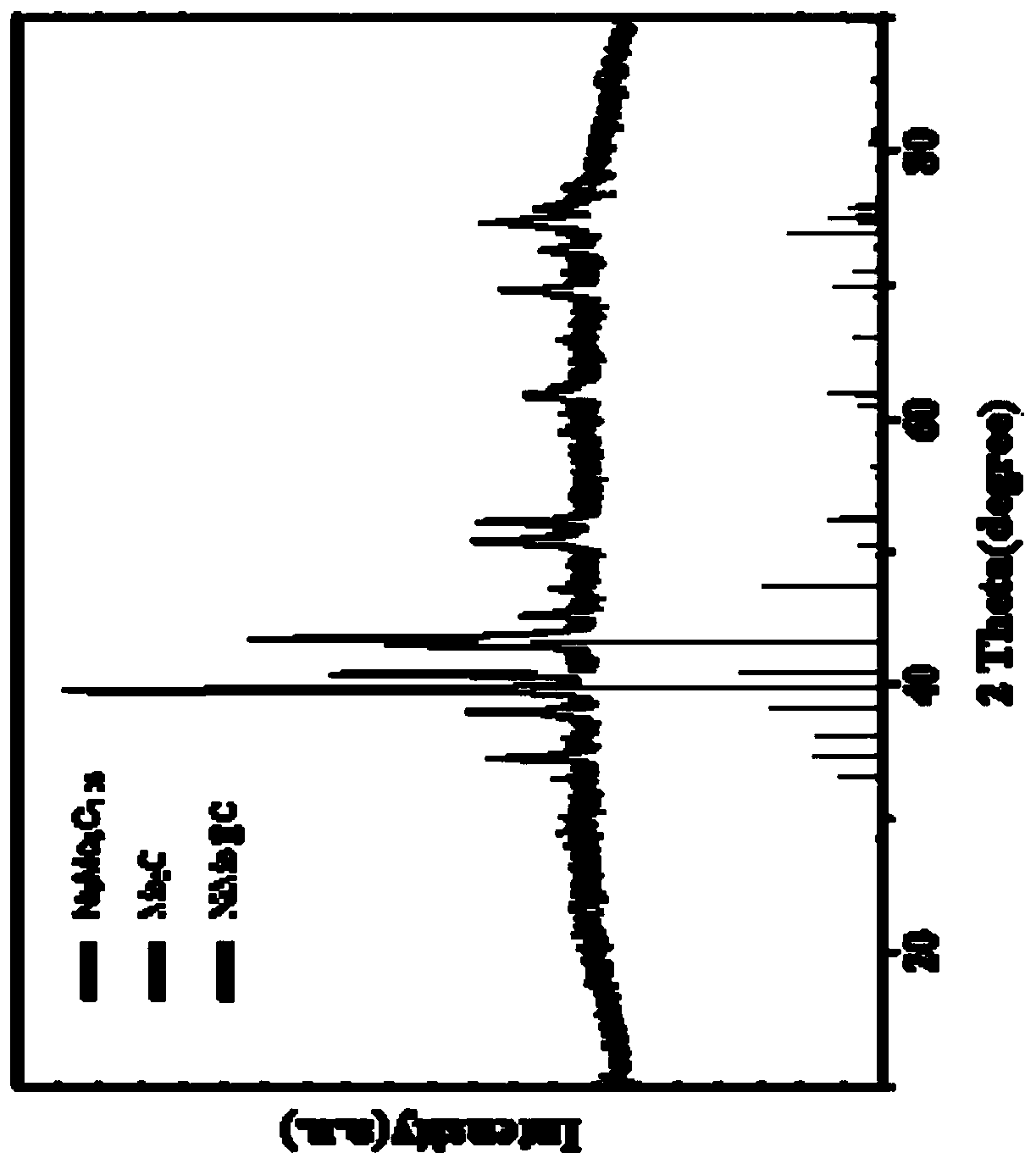
[0034] An N / O double-doped metal carbon-coated transition metal carbide nanoparticle composite material contains Mo, Ni, C, N, and O elements.
[0035] The above-mentioned N / O double-doped metal carbon-coated transition metal carbide nanoparticle composite material preparation method specifically includes the following steps:
[0036] (1) Weigh 10g of urea and dissolve it in 5ml of deionized water, adjust the pH to 6 with 0.1M HCl, stir evenly and dry at 80°C for 10h, put the dried urea in a crucible in a tube furnace under a nitrogen atmosphere Annealed at 550°C for 3 hours, cooled naturally to obtain g-C 3 N 4 ;
[0037] (2) Add 0.1g of Ni(NO 3 ) 2 ·6H 2 O and 0.05gH 8 MoN2O4 was dissolved in 5ml of deionized water to form a homogeneous solution A, 0.2g of g-C 3 N 4 Disperse in solution A, freeze-dry after ultrasonication for 5h to obtain NiMo@C precursor;
[0038] (3) The NiMo@C precursor was mixed with the same mass of zinc powder and ground evenly, then placed in...
Embodiment 2
[0040] (1) Weigh 10g of urea and dissolve it in 5ml of deionized water, adjust the pH to 6 with 0.1M HCl, stir evenly and dry at 80°C for 10h, put the dried urea in a crucible in a tube furnace under a nitrogen atmosphere Annealed at 550°C for 3 hours, cooled naturally to obtain g-C 3 N 4 ;
[0041] (2): 0.1g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 5ml of deionized water to form a homogeneous solution A, and 0.2g of g-C 3 N 4 Disperse in solution A, freeze-dry after ultrasonication for 5h to obtain Ni@C precursor or Mo@C precursor;
[0042] (3): Mix the precursor Ni@C or Mo@C with the same mass of zinc powder and grind them evenly, then place them in a crucible and anneal at 900 °C for 3 hours in a tube furnace under a nitrogen atmosphere, and cool naturally to obtain a Ni@C precursor body.
Embodiment 3
[0044] (1) Weigh 10g of urea and dissolve it in 5ml of deionized water, adjust the pH to 6 with 0.1M HCl, stir evenly and dry at 80°C for 10h, put the dried urea in a crucible in a tube furnace under a nitrogen atmosphere Annealed at 550°C for 3 hours, cooled naturally to obtain g-C 3 N 4 ;
[0045] (2): Add 0.1g H 8 MON 2 o 4 Dissolved in 5ml of deionized water to form a homogeneous solution A, 0.2g of g-C 3 N 4 Disperse in solution A, freeze-dry after ultrasonication for 5 hours to obtain Ni@C precursor or Mo@C precursor;
[0046] (3): The precursor Mo@C was mixed with the same mass of zinc powder and ground evenly, then placed in a crucible and annealed at 900 °C for 3 hours in a tube furnace under a nitrogen atmosphere, and cooled naturally to obtain a Mo@C precursor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com