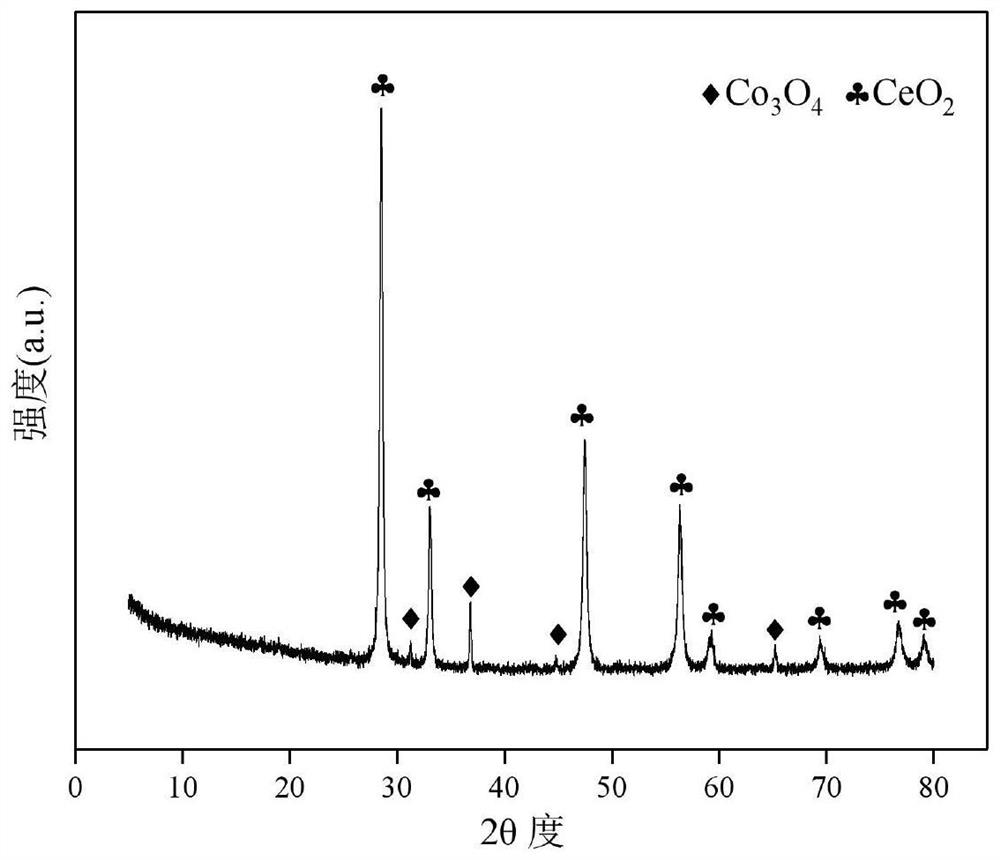
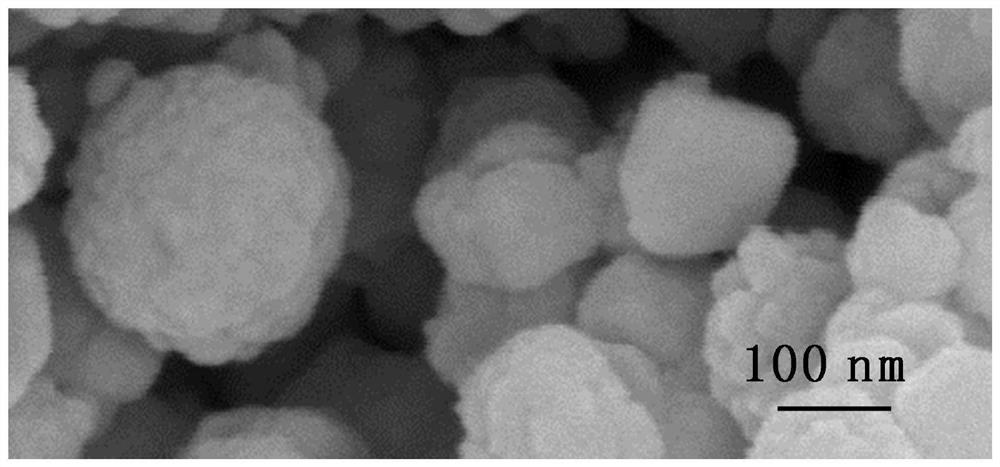
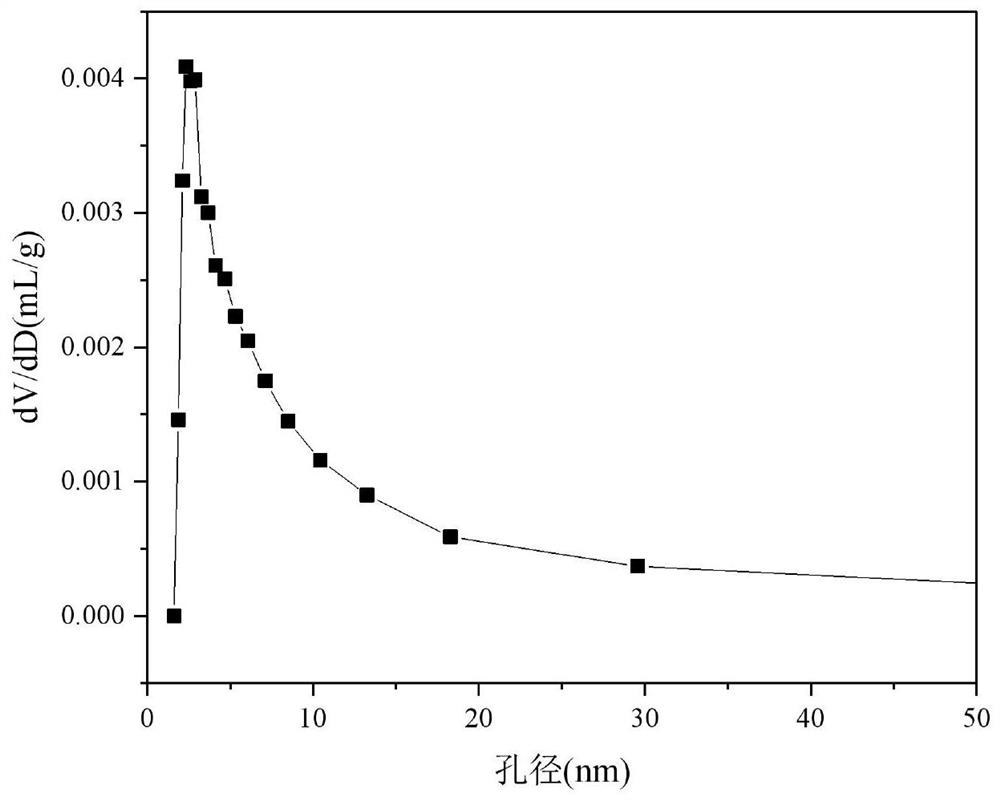
Ceria-Supported Cobalt-Based Catalysts for Autothermal Reforming of Acetic Acid to Hydrogen Production
A ceria, autothermal reforming technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, hydrogen, etc., can solve the problem of catalyst deactivation, easy to be oxidized, poor stability, etc. problems, to achieve the effect of improving the ability to resist carbon deposition, promoting gasification, and improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] Weigh 9.08g of Ce(NO 3 ) 3 ·6H 2 0 and 6.00g of urea were dissolved by adding 90.3mL and 40.1mL of deionized water respectively, mixed and stirred evenly, added 10.0mL hydrogen peroxide, stirred evenly, put into the reaction kettle, hydrothermally reacted at 240°C for 12h, and cooled to After room temperature, wash three times with deionized water; the washed sample is placed in a 140°C drying oven for 12 hours, and then the temperature is raised at a rate of 10°C / min and roasted at a temperature of 600°C for 4h to obtain CeO 2 Carrier; after weighing 1.40g of Co(NO 3 ) 2 ·6H 2 O, 6.00g of urea, respectively add 90.5mL and 40.3mL of deionized water to dissolve, mix and stir evenly, add 10.0mL hydrogen peroxide, and then add the aforementioned 3.60g of CeO 2 The carrier, stirred evenly, was put into the reaction kettle, hydrothermally reacted at 240°C for 12h, cooled to room temperature, and washed with deionized water three times; the washed samples were dried in a...
Embodiment 1
[0036] Weigh 8.07g of Ce(NO 3 ) 3 ·6H 2 0 and 6.00g of urea were dissolved in 90.5mL and 40.3mL of deionized water respectively, mixed and stirred evenly, and 10.0mL of hydrogen peroxide was added, stirred evenly, put into a reactor, hydrothermally reacted at 240°C for 12h, and cooled to After room temperature, wash three times with deionized water; the washed sample is placed in a 140°C drying oven for 12 hours, and then the temperature is raised at a rate of 10°C / min and roasted at a temperature of 600°C for 4h to obtain CeO 2 carrier; after weighing 2.80g of Co(NO 3 ) 2 ·6H 2O, 6.00g of urea, respectively add 90.0mL and 40.2mL of deionized water to dissolve, mix and stir evenly, add 10.0mL hydrogen peroxide, and then add the aforementioned 3.20g of CeO 2 The carrier, stirred evenly, was put into the reaction kettle, hydrothermally reacted at 240°C for 12h, cooled to room temperature, and washed with deionized water three times; the washed samples were dried in a 140°C ...
Embodiment 2
[0039] Weigh 7.06g of Ce(NO 3 ) 3 ·6H 2 0 and 6.00g of urea were dissolved in 90.1mL and 40.4mL of deionized water respectively, mixed and stirred evenly, and 10.0mL of hydrogen peroxide was added, stirred evenly, put into a reactor, hydrothermally reacted at 240°C for 12h, and cooled to After room temperature, wash three times with deionized water; the washed sample is placed in a 140°C drying oven for 12 hours, and then the temperature is raised at a rate of 10°C / min and roasted at a temperature of 600°C for 4h to obtain CeO 2 carrier; after weighing 4.20g of Co(NO 3 ) 2 ·6H 2 O and 6.00g of urea were dissolved by adding 90.0mL and 40.2mL of deionized water respectively, after mixing and stirring evenly, 10.0mL of hydrogen peroxide was added, and then 2.80g of CeO 2 The carrier, stirred evenly, was put into the reaction kettle, hydrothermally reacted at 240°C for 12h, cooled to room temperature, and washed with deionized water three times; the washed samples were dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com