Synthetic method of eribulin intermediate ER806047
A synthesis method and intermediate technology, which are applied in the synthesis field of Eribulin intermediate ER806047, can solve the problems of difficult to completely remove sulfur-containing by-products, increase the usage of Pd/C, and be difficult to realize industrialization, etc., so as to avoid catalysts. Poisoning risk, low production cost, and the effect of reducing the generation of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
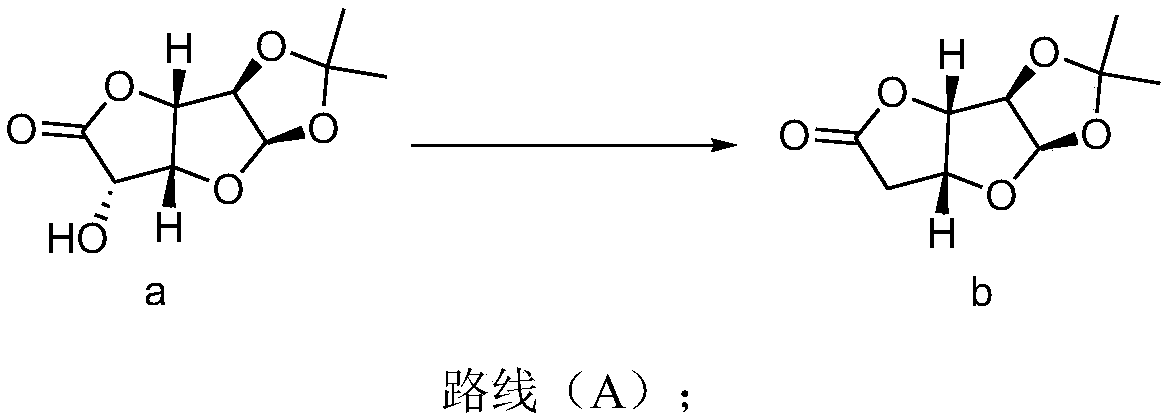
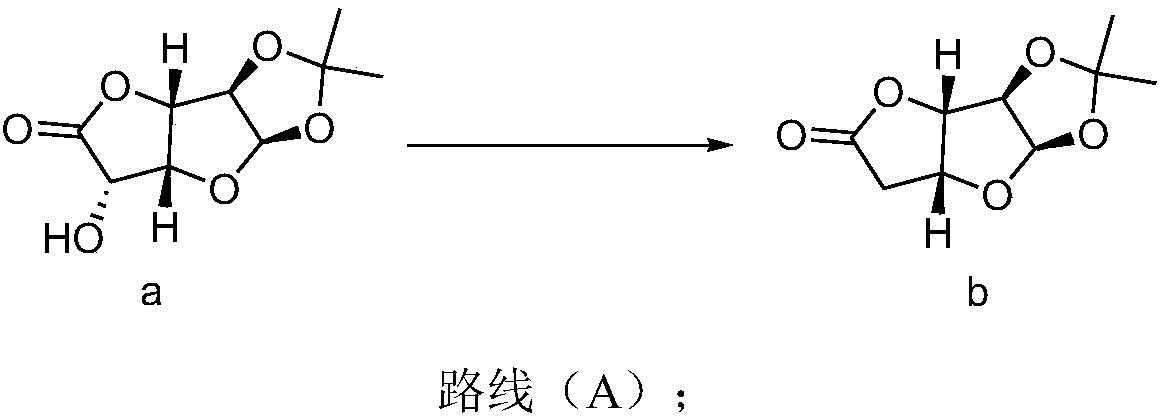
[0035] Synthesis of compound b:
[0036] plan 1:
[0037] Under the protection of nitrogen, add 1,2-dichloroethane (72L, 12V), pyridine (2.19kg, 27.75mol), triphenylphosphine (10.92kg, 42.63mol) into a 100L reactor, heat up and reflux to distill off the solvent 12L, then cooled to 25°C, then added 1,2-O-isopropylidene-α-D-glucuronic acid-6,3-lactone (6kg, 27.75mol), I 2 (3.52kg, 13.88mol), heated and refluxed for 11 hours, detected by HPLC or TLC, and the reaction ended. Cool down to 25°C, add saturated aqueous sodium thiosulfate solution (18L, 3V), let stand for liquid separation, extract the aqueous phase with 1,2-dichloroethane (6L, 1V) twice, combine the organic phases, and depressurize Concentrate to 2V (12L), perform flash column chromatography, and the eluent is petroleum ether / ethyl acetate to obtain the compound of formula b (4.44kg, 22.2mol).
[0038] 1 H-NMR (400MHz, CHLOROFORM-d 6 )δppm 1.36(s,3H)1.52(s,3H)2.66-2.80(m,2H)4.84(dd,J=10.39,3.55Hz,2H)4.96-5.06(m,1...
specific Embodiment
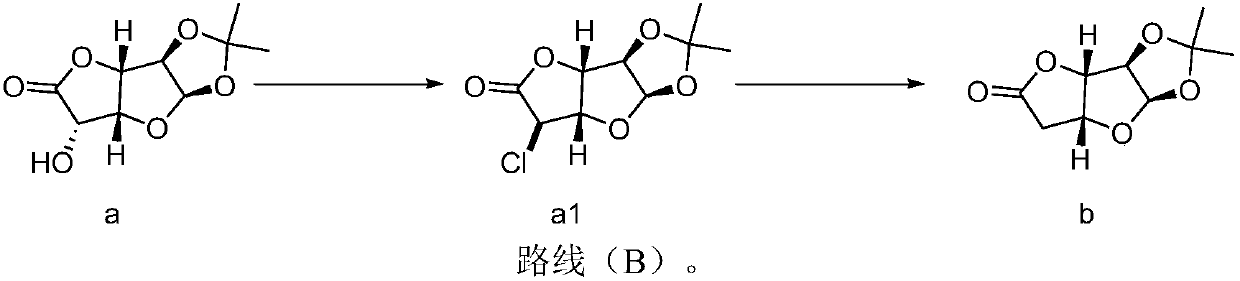
[0077] Synthesis of compound a1:
[0078] Under nitrogen protection, compound a 1,2-O-isopropylidene-α-D-glucurono-6,3-lactone (50g, 0.231mol) was dissolved in dichloromethane (150ml, 15v), Pyridine (73.08g, 0.925mol) was added, the temperature of the system was cooled to -10~-5°C, and a solution of sulfonyl chloride (38.5g, 0.324mol) in DCM (100ml, 2V) was added dropwise. After the dropwise addition, firstly keep it warm at -10-5°C for 1 hour, then keep it at 0-5°C for 1 hour, and finally raise it to about 25°C and keep it warm for 1 hour. HPLC / TLC detects that the reaction of raw materials is complete. Then the reaction system was cooled to 0-15°C, and the reaction system was slowly added to a saturated aqueous solution of sodium bicarbonate (500ml, 10V), separated, the organic phase was washed with saturated brine (100ml, 2V), and concentrated to dryness at 30°C to obtain Compound a1 (50 g, 0.21 mol), the yield was 92.1%, and it was directly submitted to the next step.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com