17 beta-hydroxysteroid hydroxylase 3 mutant enzyme, coding gene and engineering bacteria
A technology of hydroxysteroids and mutant enzymes, applied in the field of enzyme engineering and bioengineering, can solve the problems of low enzyme activity, unfavorable large-scale preparation, and low expression, and achieve the effect of improving catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
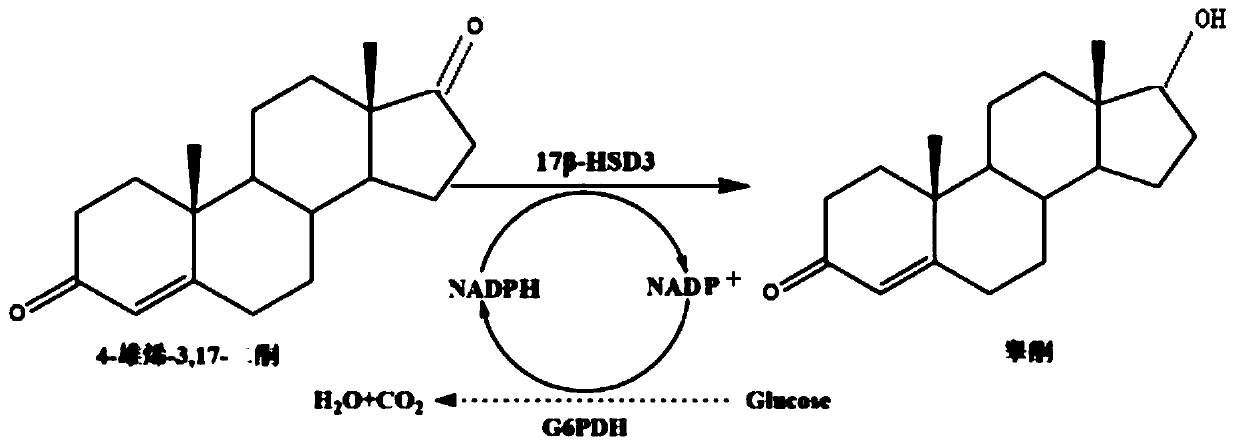
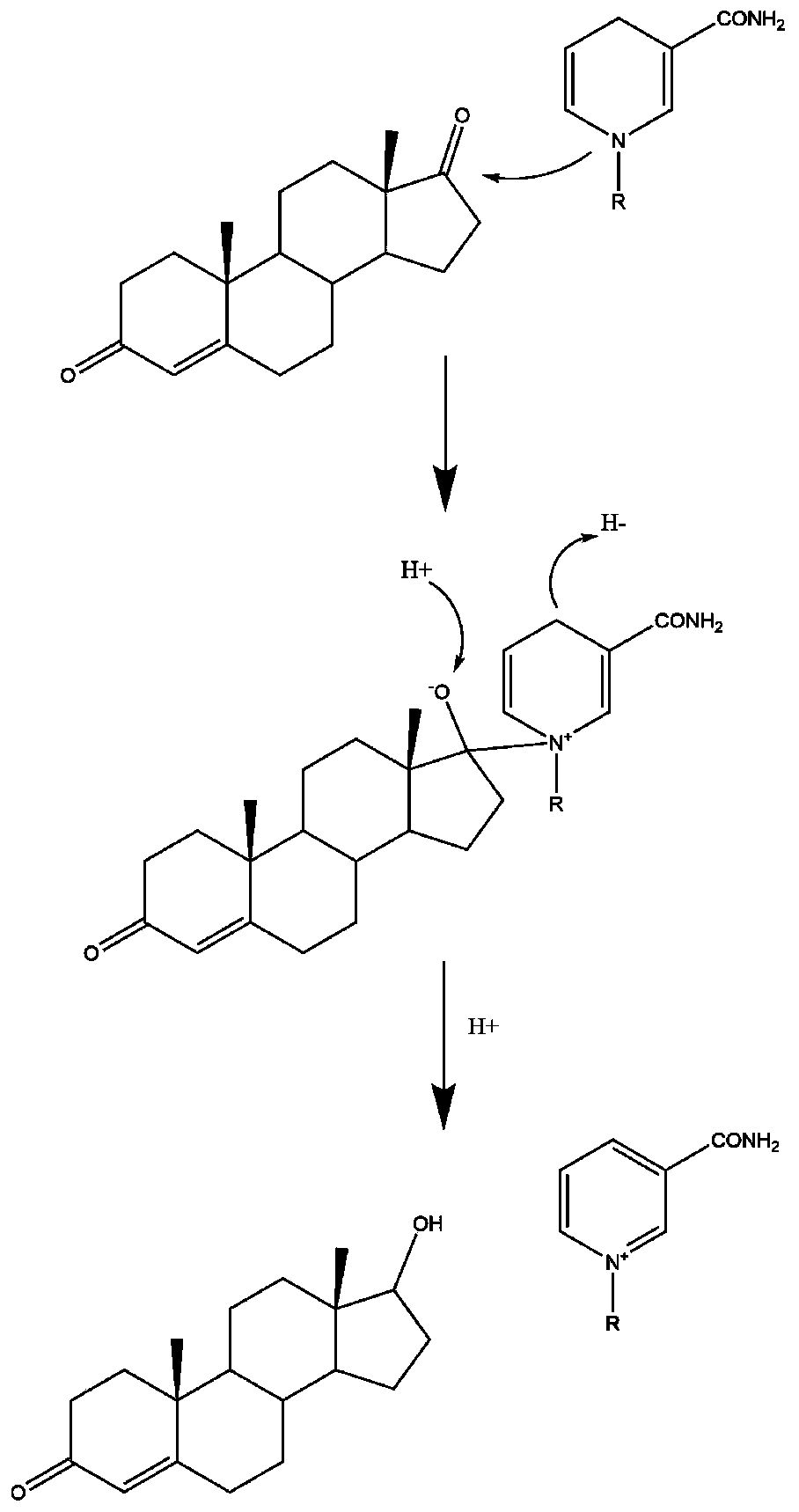
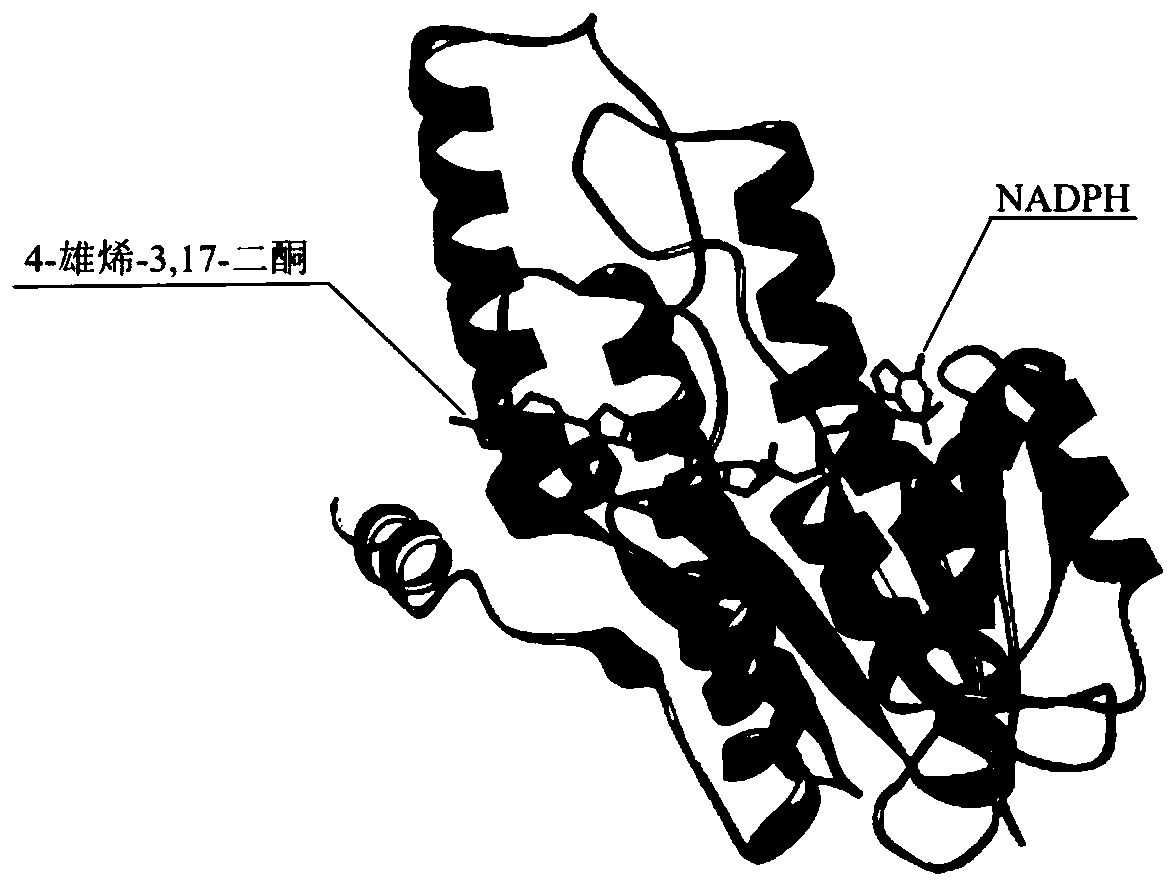
[0030] Rational Design of Mutation Sites of 17β-Hydroxysteroid Hydroxylase 3
[0031] (1) Simulation of the spatial structure of 17β-hydroxysteroid hydroxylase 3: Using the same family enzyme 17β-hydroxysteroid hydroxylase 1 (PDB: 1FDT) as a model, it was found that its structure has a set of typical features: ① consists of 8 α-helices and 8 β sheets constitute a barrel-shaped structure; ②The nicotinamide ring of NADPH and the nearby 17 residues form an oval pocket at the C-terminus; ③Two channels are found from the crystal structure for substrate entry and product release;④ Key amino acids Phe306 and Trp227 play an important role in substrate recognition. Combined with the above information, the SWISS-MODEL online server was used to simulate the spatial structure of 17β-hydroxysteroid hydroxylase 3 (SEQ ID NO.3); the structural model of homology modeling was evaluated and optimized by Verify_3D to obtain a more accurate Analog structure. From this, the rational design idea ...
Embodiment 2
[0035] Preparation of recombinant plasmids pPIC3.5K-17β-HSD3 and pPICZαA-G6PDH
[0036] A recombinant plasmid pPIC3.5K-17β-HSD3 is obtained by linking the codon-optimized 17β-hydroxysteroid dehydrogenase gene (SEQ ID NO.4) to the pPIC3.5K plasmid.
[0037] A recombinant plasmid, pPICZαA-G6PDH, will be able to catalyze NADP + The enzyme for synthesizing NADPH, such as the glucose-6-phosphate dehydrogenase gene (SEQ ID NO. 18) derived from Saccharomyces cerevisiae, is obtained by linking to pPICZαA plasmid.
[0038] Wherein, the source of the 17β-hydroxysteroid dehydrogenase gene: Homo sapiens (GI: 21706851), the nucleotide sequence of the gene is shown in SEQ ID NO.17; the human source 17β-hydroxysteroid hydroxylase 3 After codon optimization of the gene, a codon-optimized 17β-hydroxysteroid dehydrogenase gene (SEQID NO.4) suitable for expression in Pichia pastoris host was obtained;
[0039]The pPIC3.5K plasmid and pPICZαA plasmid were respectively purchased from Thermo Fish...
Embodiment 3
[0055] Contains a mutant enzyme encoding 17β-hydroxysteroid hydroxylase 3 (17β-HSD3 G186R / Y195W ) gene recombinant plasmid (abbreviation: recombinant plasmid containing mutant gene) preparation
[0056] Based on the mutation site rationally designed in Example 1, the primers for site-directed mutagenesis were designed and synthesized as follows:
[0057] G186R-F: (introducing a mutation at position 186) (SEQ ID NO.13)
[0058] G186R-R: (introducing a mutation at position 186) (SEQ ID NO.14)
[0059] Y195W-F: (Introduction of mutations at positions 186 and 195) (SEQ ID NO.15)
[0060] Y195W-R: (Introduction of mutations at positions 186 and 195) (SEQ ID NO.16)
[0061] According to the requirements of the seamless recombination kit, the recombinant plasmid containing the mutant gene was constructed by seamless connection and reverse PCR technology, which is mainly divided into two steps: (1) using the recombinant plasmid pPIC3.5K-17β-HSD3 as a template, G186R-F ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com