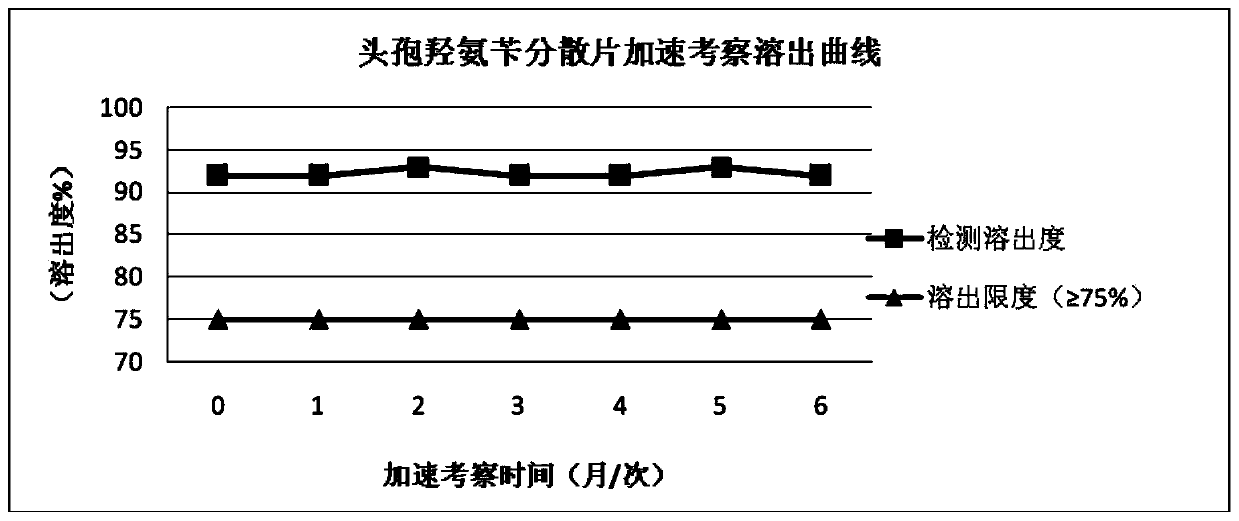
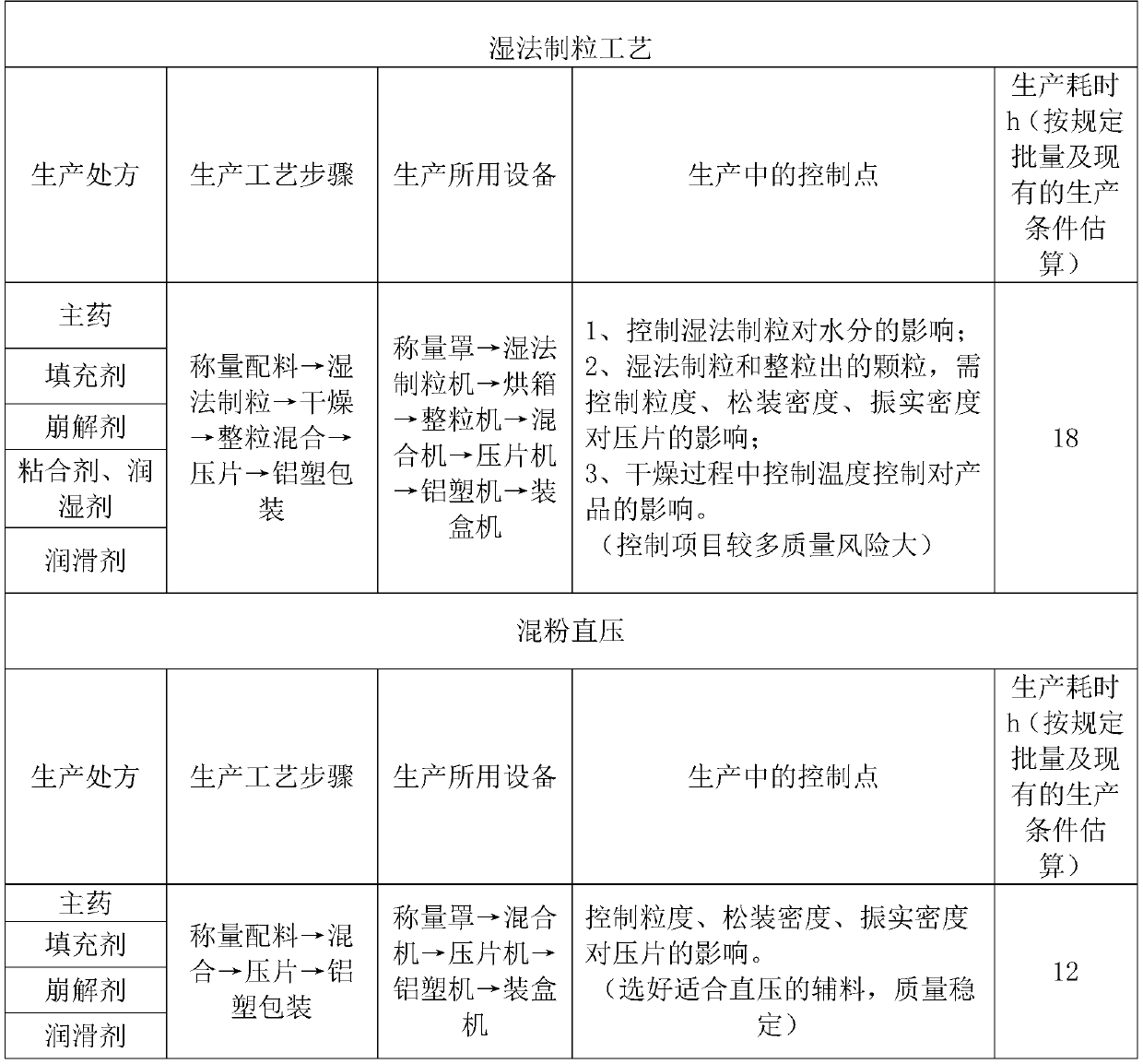
Cefadroxil dispersible tablets and preparation method therefor
A technology for cefadroxil dispersible tablets and cefadroxil, which is applied to medical preparations without active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of long production cycle, degradation risk of cefadroxil, The problem of high energy consumption, to achieve the effect of easy operation, suitable for large-scale production, and uniform drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The prescription composition of every 10000 tablets of preparations: cefadroxil 5000g, ordinary microcrystalline cellulose 1000g, lactose 220g, starch 550g, magnesium stearate 80g.
[0023] Preparation:
[0024] After the raw materials are all passed through a 30-mesh sieve, take cefadroxil, common type microcrystalline cellulose, lactose and starch according to the prescription amount, mix uniformly, then add magnesium stearate, and after mixing uniformly, obtain the mixed material, the mixed material The bulk density is 0.5g / cm 3 , tap density 0.8g / cm 3 , after passing the test, the mixed material is compressed into tablets by a tablet machine to obtain cefadroxil dispersible tablets, and the hardness of the tablet is 110N.
Embodiment 2
[0026] The prescription composition of every 10000 preparations: cefadroxil 5000g, microcrystalline cellulose (PH101) 1110g, mannitol 190g, carboxymethyl starch sodium 500g, magnesium stearate 60g.
[0027] Preparation:
[0028] After the raw materials are all passed through a 30-mesh sieve, take cefadroxil, microcrystalline cellulose PH101, mannitol and carboxymethyl starch sodium according to the prescription amount, mix them uniformly, then add magnesium stearate, and mix them uniformly to obtain the mixed material. The bulk density of the material is 0.5g / cm 3 , tap density 0.9g / cm 3 , after passing the test, the mixed material is compressed into tablets by a tablet machine to obtain cefadroxil dispersible tablets, and the hardness of the tablet is 120N.
Embodiment 3
[0030] The prescription composition of every 10000 preparations: cefadroxil 5000g, microcrystalline cellulose (PH102) 1100g, starch 220g, carboxymethyl starch sodium 500g, magnesium stearate 30g.
[0031] Preparation:
[0032] After the raw materials are all passed through a 30-mesh sieve, take cefadroxil, microcrystalline cellulose PH102, starch and carboxymethyl starch sodium according to the prescription quantity, mix uniformly, then add magnesium stearate, after mixing uniformly, obtain the mixed material, mix The bulk density of the material is 0.6g / cm 3 , tap density 0.9g / cm 3 , after passing the test, the mixed material is compressed into tablets by a tablet machine to obtain cefadroxil dispersible tablets, and the hardness of the tablet is 110N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| bulk density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com