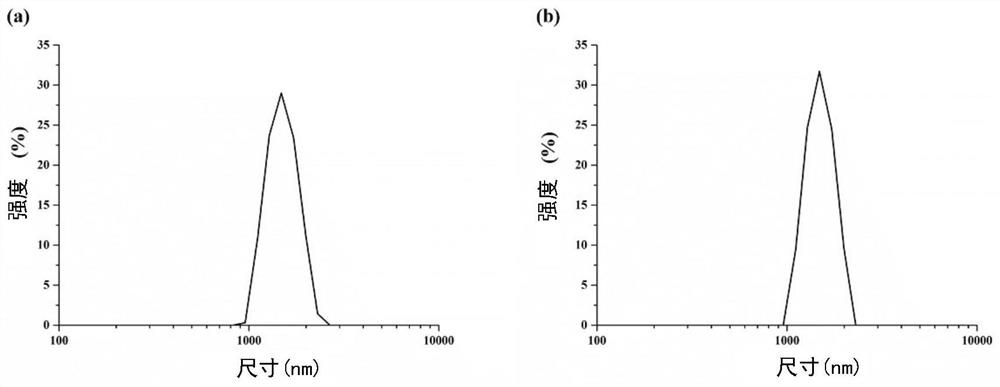
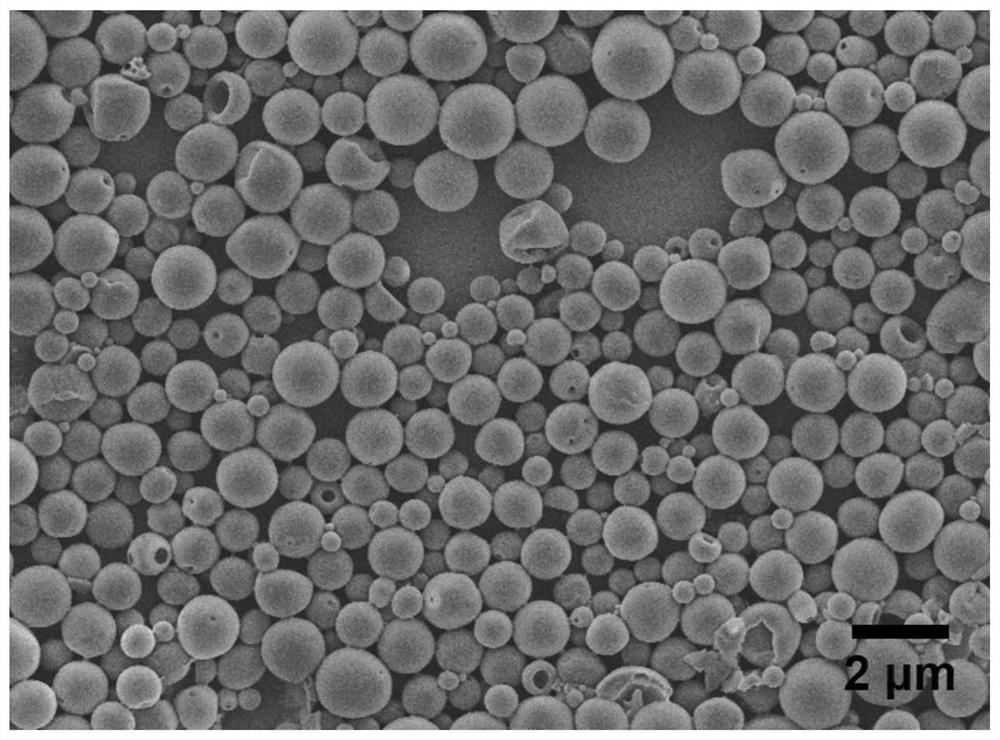
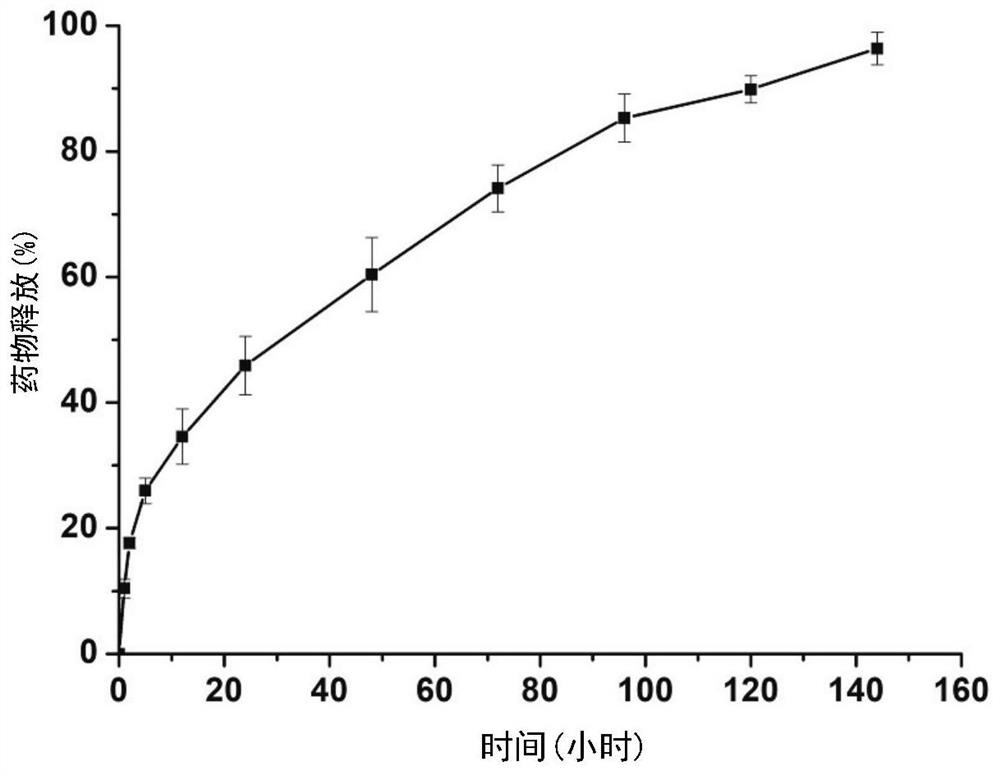
Sustained-release microsphere drug delivery system and preparation method thereof
A technology of slow-release microspheres and microspheres, which can be used in drug delivery, drug combination, pharmaceutical formulations, etc., and can solve problems such as blocked needles, patient pain, and drug storage time leakage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] According to the above requirements, one aspect of the present invention provides a method for preparing injectable and storable drug sustained-release microspheres, which comprises the steps of:
[0067] 1) preparing the water phase W of the organic stabilizer aqueous solution;
[0068] 2) dissolving the water-soluble drug in the water phase to obtain a drug solution S;
[0069] 3) dissolving the biodegradable polymer material in an organic solvent to obtain the oil phase O;
[0070] 4) mixing the drug solution S and the oil phase O, and emulsifying to obtain an S / O type primary emulsion;
[0071] 5) inject the primary emulsion S / O obtained in step 4) into the water phase W prepared in step 1, and emulsify to obtain the S / O / W type secondary pre-emulsion;
[0072] 6) homogenizing the S / O / W pre-emulsion obtained in step 5), wherein the S / O / W pre-emulsion can optionally be injected into the aqueous phase W obtained in step 1);
[0073] 7) Optionally volatilize the orga...
Embodiment 1
[0207] Synthesis and characterization of embodiment 1 polylactic acid-polyethylene glycol (PLA-PEG)
[0208] Material: Polyethylene glycol methyl ether (mPEG-OH, purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.)
[0209] D, L-lactide (purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.)
[0210] 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., English full name is 1,8-Diazabicyclo[5.4.0]undec -7-ene)
[0211] Benzoic acid (purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.)
[0212] All other reagents were of analytical grade and purchased from Shanghai Titan Technology Co., Ltd.
[0213] Method: Dissolve mPEG-OH (0.08mmol, 0.4g) in a round bottom flask filled with 10mL of anhydrous dichloromethane, add D,L-lactide (19.41mmol, 2.8g) and DBU (0.19mmol, 185.5 μL), the round bottom flask was sealed, and the reaction was stirred at room temperature. After 1 h, the polymerizati...
Embodiment 2
[0215] Example 2 Preparation of injectable storable ropivacaine hydrochloride sustained-release microspheres
[0216] Material: PLA-PEG prepared in Example 1 or commercially available (such as: Jinan Daigang Biological Engineering Co., Ltd.)
[0217] Ropivacaine hydrochloride (purchased from Shanghai Macklin Biochemical Technology Co., Ltd.)
[0218] Polyvinyl alcohol (Mw ~ 27,000, purchased from Shanghai Macklin Biochemical Technology Co., Ltd.)
[0219] All other reagents were of analytical grade and purchased from Shanghai Titan Technology Co., Ltd.
[0220]Method: Dissolve 4mg of ropivacaine hydrochloride in 1ml of 0.2% polyvinyl alcohol aqueous solution to obtain inner water phase W1, dissolve 40mg of PLA-PEG in 2ml of dichloromethane to form oil phase O; prepare 0.5% polyethylene Alcohol aqueous solution is used as the external water phase W2; the obtained ropivacaine hydrochloride solution W1 is injected into the oil phase O, and ultrasonically mixed (Q700, Qsonica, U...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com