Synthesis method of s-triazine boronic acid pinacol ester
A synthesis method and technology of boric acid, which are applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., can solve problems affecting the performance of photoelectric materials, failure to obtain final products, and difficulties in the preparation of boric acid, etc. , to achieve the effect of simple and effective treatment, thorough response and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
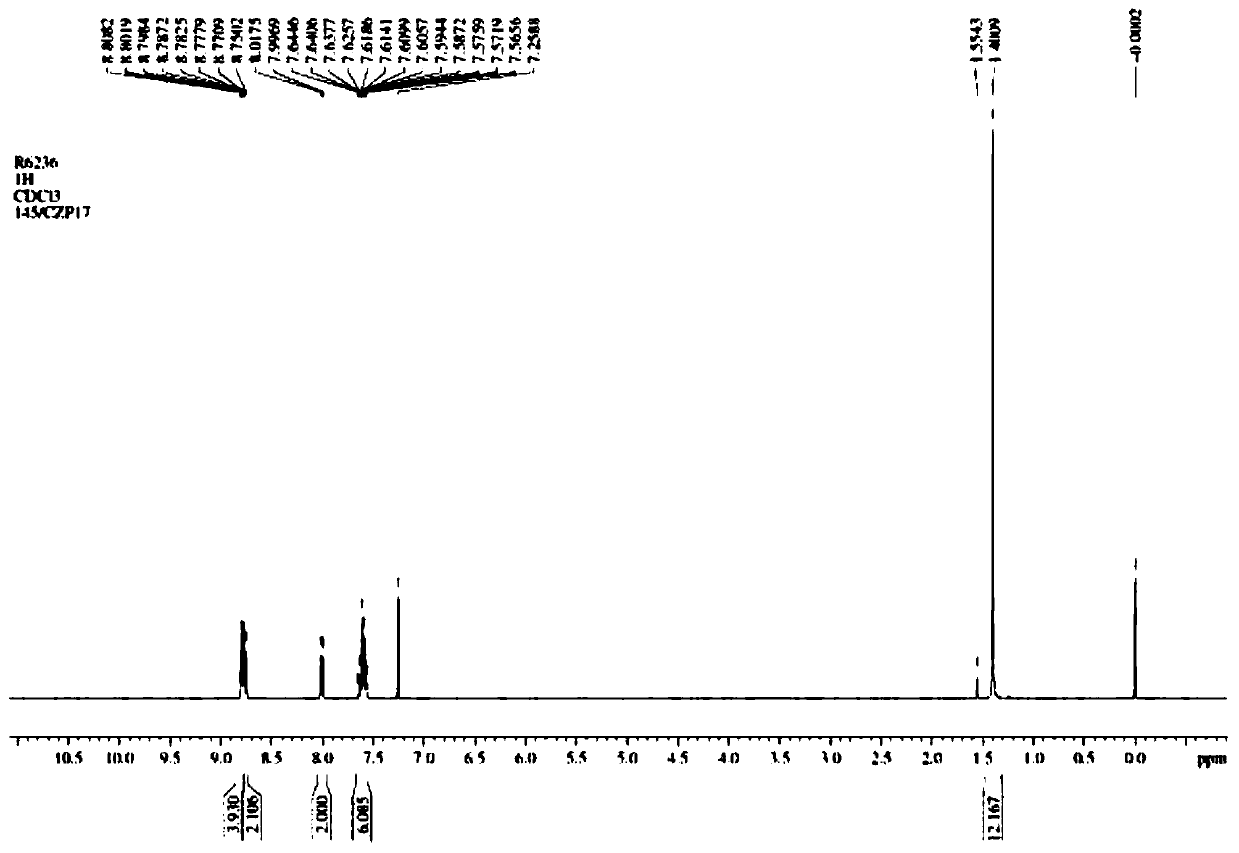
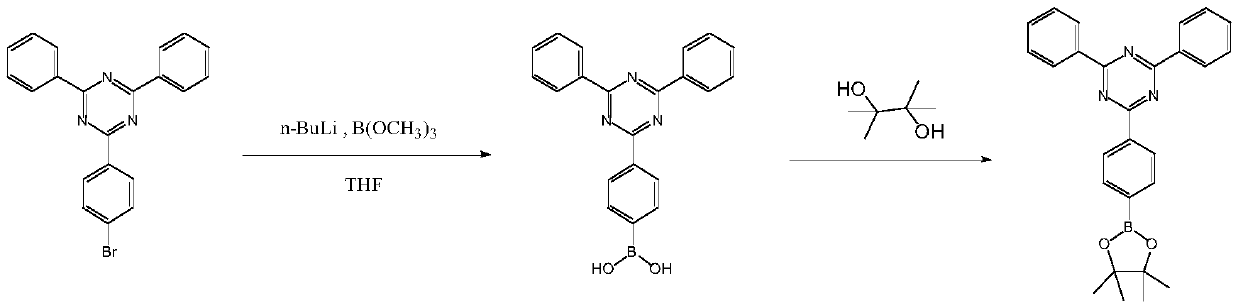
[0016] (1) Put 38.83g (0.1mol) of 2-(4-bromophenyl)-4,6-diphenyl-1,3,5-triazine and 260mL of tetrahydrofuran into the reactor, stir until completely dissolved, Add 85 mL of diethoxymethane, replace with nitrogen for 30 minutes, pass through liquid nitrogen, use an anhydrous ethanol bath to lower the temperature of the reaction system to below -90°C, and slowly add n-butyl lithium in n-hexane dropwise through a constant pressure funnel while maintaining this temperature Solution 50mL (2.5mol / L), keep this temperature for 30min after adding, then slowly add 18.18g of trimethyl borate dropwise through constant pressure funnel, keep this temperature for 30min after adding, naturally warm to room temperature. Add dilute hydrochloric acid with a mass percentage of 15% to adjust the pH to 6-7, separate the water layer, extract the water layer with ethyl acetate twice, each time with 15 mL, combine the organic layers, and dry the organic layer with anhydrous magnesium sulfate for 1 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com