Method for panning and multiplication culture of liver stem cells and application thereof
A liver stem cell and culture medium technology, applied in the field of bioengineering, can solve problems such as defects in the differentiation potential of liver stem cell populations, and achieve excellent effects and clear ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Example 1 Preparation of culture medium
[0113] The medium components include:
[0114] The liquid basic medium is RPMI1640, 1% N2, 1% B27, 1% insulin-transferrin-sodium selenite (ITS) mixture, 1% glutaminase substitute, 10mM nicotinamide, 50ng / mL epidermis Growth factors, 20ng / mL bone morphogenetic factor-4, 100ng / mL fibroblast growth factor-10, 100ng / mL RSPO1, 3μM CHIR99021, 5μM A83-01, 10μM Y-27632.
Embodiment 2
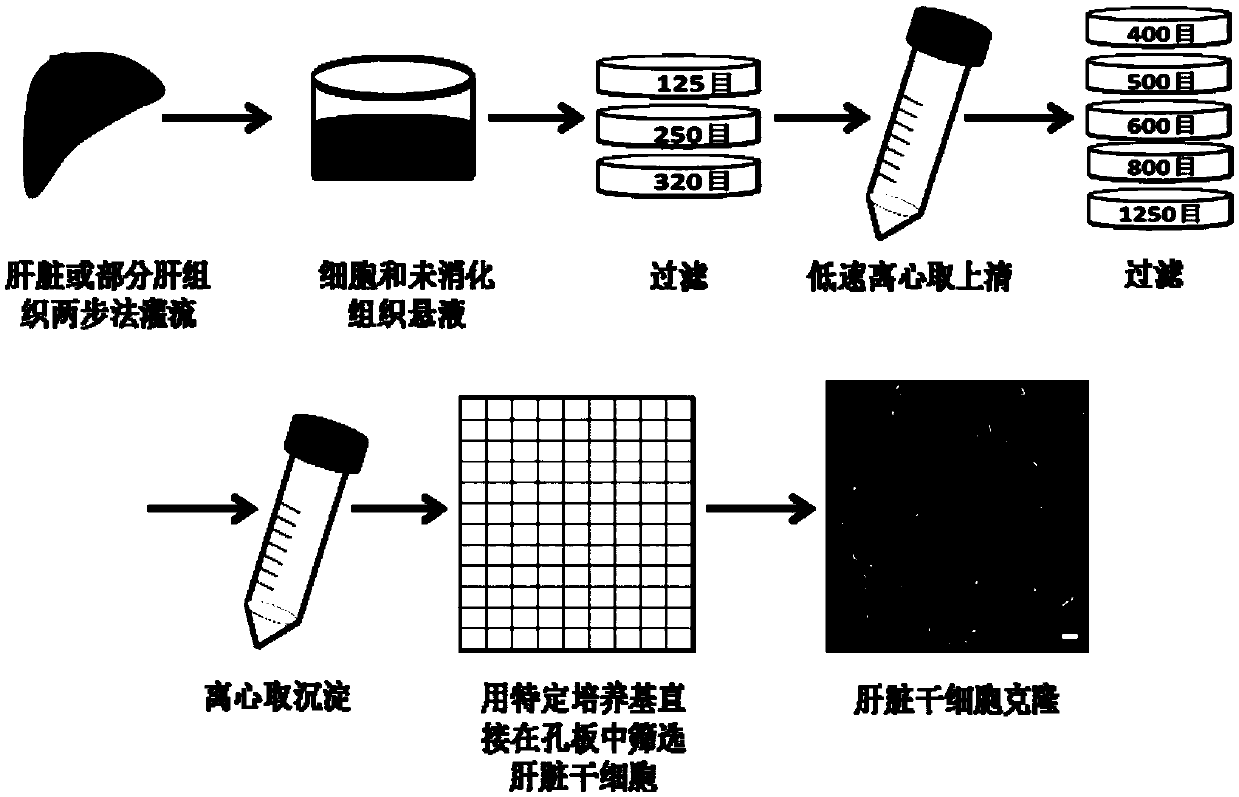
[0115] Example 2 Screening of liver stem cells
[0116] 1.1 Two-step perfusion of mouse liver
[0117] 1)Constant flow pump pretreatment: the pipes of the constant flow pump are first perfused with absolute ethanol, then cleaned with autoclaved deionized water, and finally cleaned with D-Hanks for use;
[0118] 2) Experimental mouse anatomy: After anesthetizing the mouse with 20μL of avertin per gram of body weight by intraperitoneal injection, the mouse is fixed on the dissecting board, the mouse's thoracic cavity is cut, the inferior vena cava is found, and the disposable indwelling needle Insert it and fix it with surgical sutures; 3mL of anticoagulant is injected into the liver of the mouse through the indwelling needle;
[0119] 3) Mouse liver perfusion: first adjust the speed of the constant flow pump to 5rpm / min, then start the perfusion with D-Hanks; cut off the portal vein, ligate the superior vena cava, time for 15 minutes and adjust the speed of the constant flow pump to 20...
Embodiment 3
[0140] Example 3 Establishment and expansion of liver stem cells
[0141] 1. Experimental method
[0142] 1.1 Establishment of liver stem cells
[0143] 1) Liver non-parenchymal cells are cultured in panning medium for 4-7 days;
[0144] 2) Use a self-made elbow glass needle to gently scrape the epithelioid clones with uniform cell morphology and cell number greater than 50 and transfer to a 48-well cell culture well plate coated with 1% Matrigel for 3 days;
[0145] 3) Replace the fresh panning medium containing 1x double antibody, after that, change the medium every two days;
[0146] 4) When the cells grow to 80% confluence, they are successively passaged to 24-well plates, 12-well plates and 6-well plates at a ratio of 1:2.
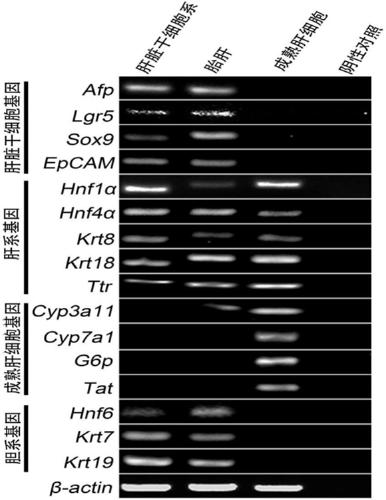
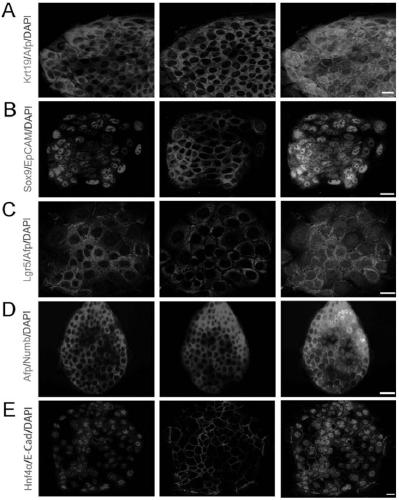
[0147] 1.2 Identification of the division mode of established liver stem cells (PHK26 staining)
[0148] 1) The cells are digested into single cells with Accutase (Sigma);
[0149] 2) Resuspend fine cells in appropriate medium, count on hemocytometer, take 5×10 5 c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com