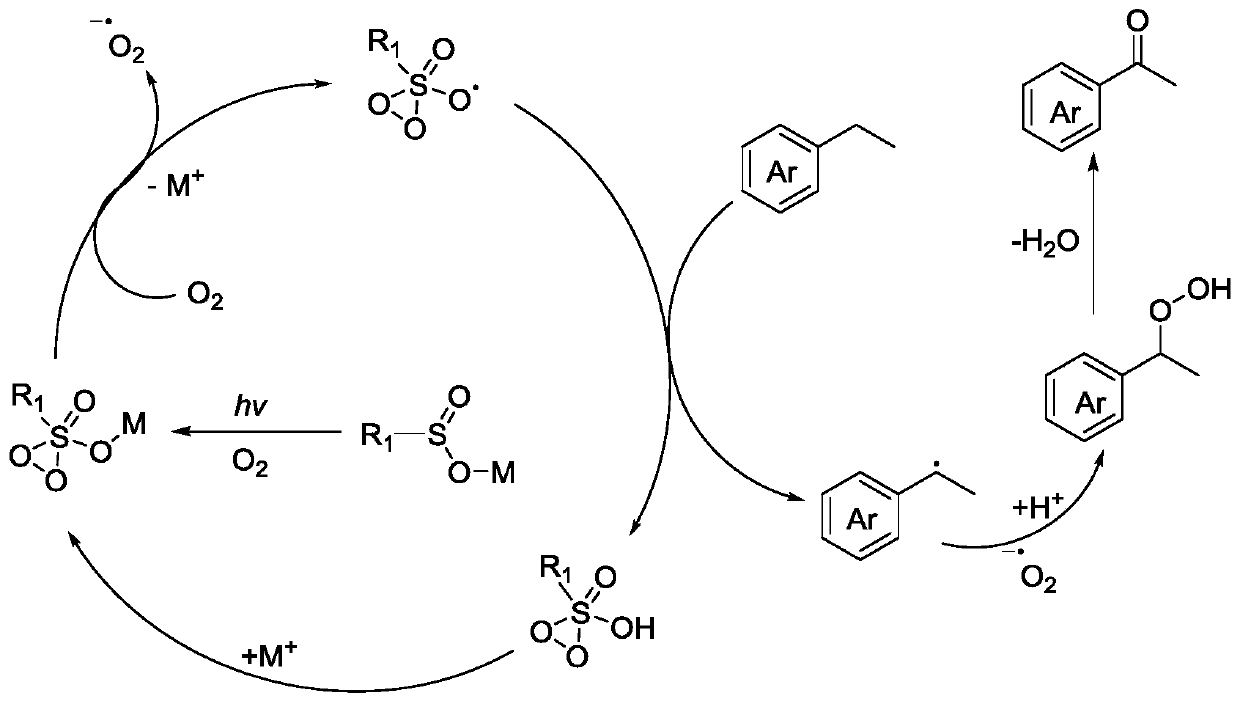
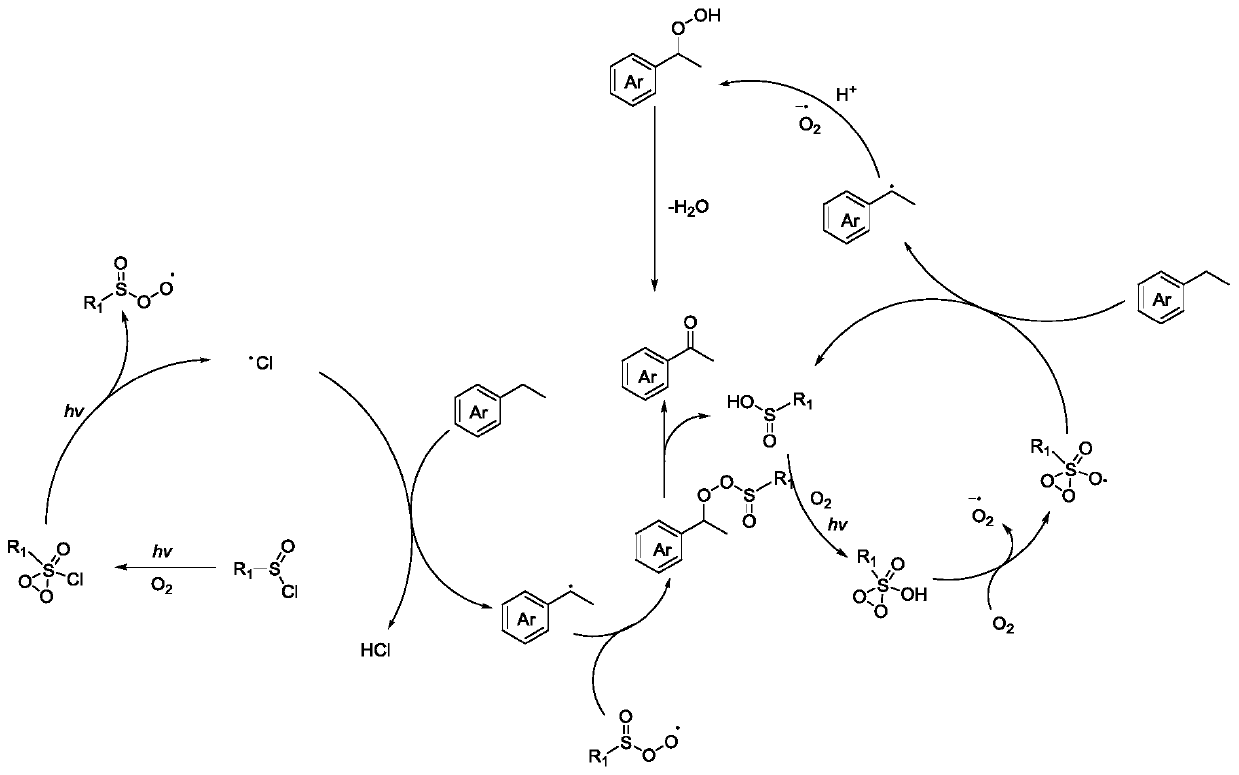
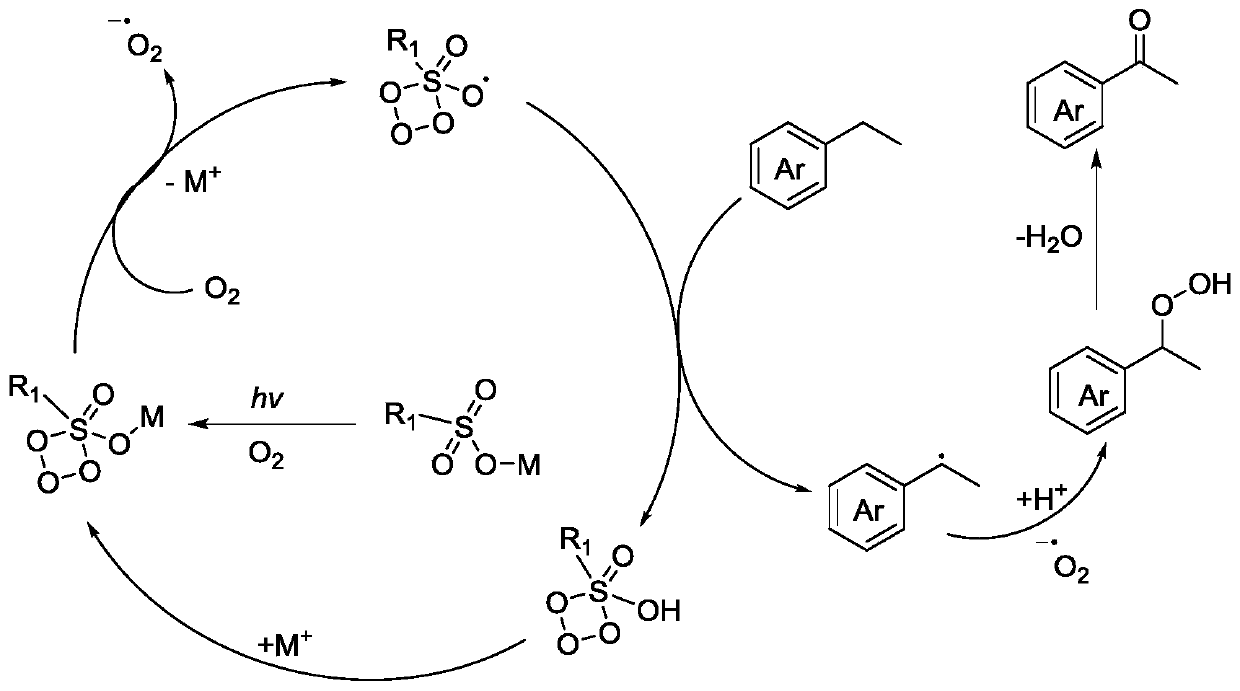
Aerobic oxidation system containing sulfinic acid, sulfonic acid or derivative thereof and photo-catalytic oxidation method thereof
A technology of aerobic oxidation and derivatives, which is applied in the fields of chemical technology, materials and biomedicine, can solve problems such as limited application, environmental pollution, and inability of products to stay, and achieve reduced production costs, energy consumption, equipment investment, and overall The effect of reducing operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0284] The reactant toluene (0.2mmol) and the auxiliary agent sodium trifluoromethanesulfinate (0.5eq) were dissolved in the organic solvent anhydrous CH 3 CN (2mL) to obtain the reaction mixture, the reaction mixture is placed in a reaction tube containing a magnetic stirrer, and then the reaction tube is placed under 34W CFL or blue light (wavelength is 400-455nm) (wherein the reaction mixture The liquid is placed under the reaction light source), and the light reaction is carried out at room temperature, air or oxygen atmosphere (1atm) until the reaction is complete (about 12h). After the reaction was completed, the reaction mixture was concentrated under reduced pressure to obtain the crude product of the target compound, which was separated by column chromatography to obtain the target compound benzaldehyde or quantified by GC-MS (yield 23%).
[0285] The characterization results are: 1 H NMR (800MHz, CDCl 3 )δ10.03(s, 1H), 7.89(d, J=7.0Hz, 2H), 7.64(t, J=8.5Hz, 1H), 7....
Embodiment 2
[0289] Example 2 adopts the same method as Example 1, the difference is only the auxiliary agent, which is specifically listed in Table 1a.
[0290] The specific reaction conditions of table 1a embodiment 2
[0291]
[0292]
Embodiment 2a-2q
[0294] Embodiment 2a-2q adopts the same method of embodiment 1 (the time of each reaction is about more than 8 hours and can last to 12 hours or longer), and the difference is only the reactants and light conditions (wavelength of the light source), specifically listed in Table 1b.
[0295] The specific reaction conditions of table 1b embodiment 2a-2q (molecular oxygen species is O 2 )
[0296]
[0297] The characterization results for Examples 2a, 2b, 2d and 2e are listed in Table 1c.
[0298] Table 1c Characterization data for Examples 2a, 2b, 2d and 2e
[0299]
[0300] The reaction formula of embodiment 2c is as follows:
[0301]
[0302] The reaction formula of embodiment 2d is as follows:
[0303]
[0304] The reaction formula of embodiment 2e is as follows:
[0305]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com