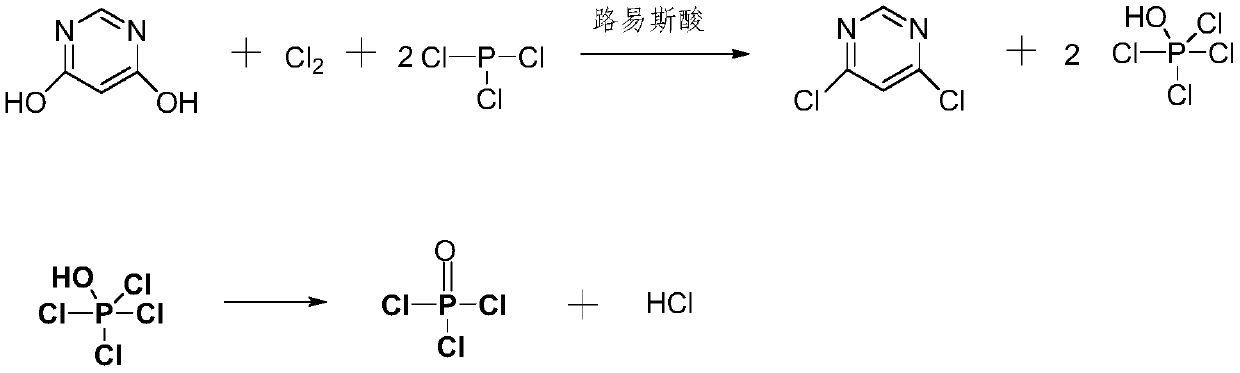
Preparation method of 4, 6-dichloropyrimidine
A technology of dichloropyrimidine and dihydroxypyrimidine, which is applied in the field of 4,6-dichloropyrimidine synthesis, can solve the problems of waste of phosphorus resources, large alkali consumption, and low yield, so as to avoid recycling and reuse, and avoid containing Phosphorus wastewater, the effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In the reaction device equipped with chlorine passing device, thermometer, reflux condenser and stirrer, add 4,6-dihydroxypyrimidine (18g, content 98%, 0.157mol), anhydrous magnesium chloride (0.5g, 99%), Phosphorus oxychloride (172g, 99.5%, 1.116mol), phosphorus trichloride (50g, 98%, 0.357mol), chlorine gas (23.5g, 99.5%, 0.329mol), control the reaction temperature at 66°C, pressure 0.1MPa After reacting for 3.5 hours, sampling analysis showed that the content of 4,6-dihydroxypyrimidine was 0.23%, and the content of 4,6-dichloropyrimidine was 99.87%. After the reaction was over, keep the temperature at 80°C and vacuum at -0.09MPa for direct distillation Obtain 162g (99.4%) of phosphorus oxychloride (99.4%) and crude 4,6-dichloropyrimidine; dissolve the crude 4,6-dichloropyrimidine in 3 times the volume of water, add sodium carbonate to adjust the pH value to 5.5, and then add the dissolved 1 times the volume of the aqueous solution of the organic solvent extraction se...
Embodiment 2
[0033] In the reaction device equipped with chlorine passing device, thermometer, reflux condenser and stirrer, add 4,6-dihydroxypyrimidine (18g, content 98%, 0.157mol), anhydrous magnesium chloride (0.5g, 99%), Toluene (100g, 99.5%, 12766mol), phosphorus trichloride (50g, 98%, 0.357mol), chlorine (23.5g, 99.5%, 0.329mol), control the reaction temperature at 68°C and the pressure of 0.3MPa for 1.5 hours, Sampling and analysis showed that the content of 4,6-dihydroxypyrimidine was 0.71%, and the content of 4,6-dichloropyrimidine was 98.76%. After the reaction was completed, the polar solvent was directly distilled off, and the temperature was kept at 75°C and the vacuum degree was at -0.09MPa. Distilled under conditions to obtain 49.62g (99.5%) of phosphorus oxychloride; add 100g of cold water to the crude 4,6-dichloropyrimidine, keep the temperature at 22°C, adjust the pH value to 6 with sodium carbonate, and extract three times with 140g of dichloromethane , combine the organ...
Embodiment 3
[0035] Add 4,6-dihydroxypyrimidine (18g, content 98%, 0.157mol), anhydrous aluminum chloride (0.5g, 99% ), anhydrous magnesium chloride (0.5g, 99%), chlorobenzene (120g, 99.8%, 1.066mol), phosphorus trichloride (50g, 98%, 0.357mol), chlorine gas (23.5g, 99.5%, 0.329mol) , control the reaction temperature at 80°C and the pressure of 0.05MPa to react for 3 hours. After the reaction is over, the content of 4,6-dihydroxypyrimidine and 4,6-dichloropyrimidine are 0.89% and 98.7%, respectively. In addition to the polar solvent, 108g (99.5%) of chlorobenzene was obtained, and the temperature was kept at 83°C, and the vacuum degree was distilled under the condition of -0.09MPa to obtain 49.07g (99.36%) of phosphorus oxychloride; in 4,6-dichloropyrimidine crude product Add 100g of cold water to the mixture, keep the temperature at 24°C, adjust the pH value to 7 with sodium hydroxide, extract three times with 140g of dichloromethane, combine the organic phase, add the organic phase dropw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com