Iron removing device and method for nickel-cobalt raffinate
A technology of raffinate and nickel-cobalt, which is applied in the field of hydrometallurgy, can solve the problems of heavy burden of follow-up process treatment, limited iron removal effect, high cost, etc., and achieves significant cost-effectiveness, reduced consumption of auxiliary materials, and strong process stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
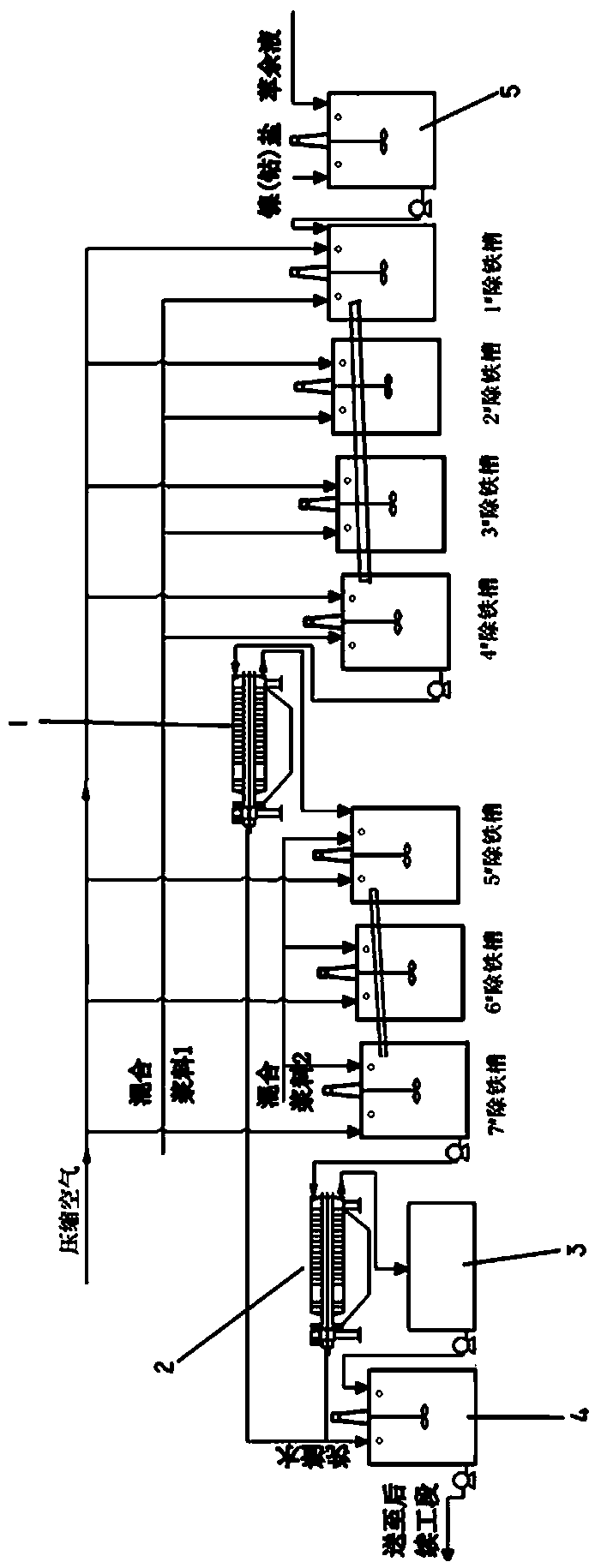
[0031] The invention provides a method for removing iron from nickel-cobalt raffinate, and the method for removing iron comprises the following steps;
[0032] Step 1, oxidation of Fe in the raffinate 2+ According to the nature of the leaching solution, select the corresponding nickel salt or cobalt salt. When Fe2+0.05-0.12g / L, the oxidation is considered complete; (if the leaching solution mainly contains Ni, choose nickel salt) as the oxidant, oxidant nickel salt or cobalt The origin of the salt is crude nickel hydroxide or hydroxide in Africa. Due to air oxidation during long-distance transportation, some nickel (cobalt) exists in a trivalent (high price) chemical state, which has strong oxidizing properties. During the reaction with raffinate, Fe can be rapidly oxidized 2+ , and a large amount of Ni is produced by leaching 2+ (Co 2+ ), thereby increasing the concentration of nickel (cobalt) metal ions in the raffinate. At the same time, because the nickel (cobalt) salt...
Embodiment 2
[0051] Embodiment 2 is the same as embodiment 1.
[0052] A certain cobalt ore leachate contains Co21g / L and total Fe5.2g / L (of which Fe 2+ 5.1g / L), Ni1.2g / L, and a small amount of other impurities.
[0053] Step 1, put an appropriate amount of crude cobalt hydroxide into the raffinate, control the reaction temperature at 50°C, and the reaction time for 5h. After the reaction is completed, Fe 2+ 0.07g / L, pH1.8.
[0054] Step 2: The iron removal section adopts 7 iron removal tanks for continuous operation with air exposure, the reaction temperature is controlled at 55°C, the total reaction time is 13 hours, and the mixed slurry with a mass concentration of 50% is used as a neutralizing agent.
[0055] Among them, the first stage adopts 4 iron removal tanks, and the slurry weight ratio quicklime:limestone=1:5; the second stage adopts 3 iron removal tanks, and the slurry weight ratio quicklime:limestone=1:1.5.
[0056] Step 3, one-stage iron removal 1 # Iron removal tank pH2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com