A chimeric antigen receptor immune cell and its preparation method and application
A chimeric antigen receptor and immune cell technology, applied in the field of genetic engineering, can solve the problem of γδT cells not being treated with coronavirus infectious diseases, and achieve the effect of avoiding off-target effects, being infected by viruses, and preventing transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1 Design of Chimeric Antigen Receptor
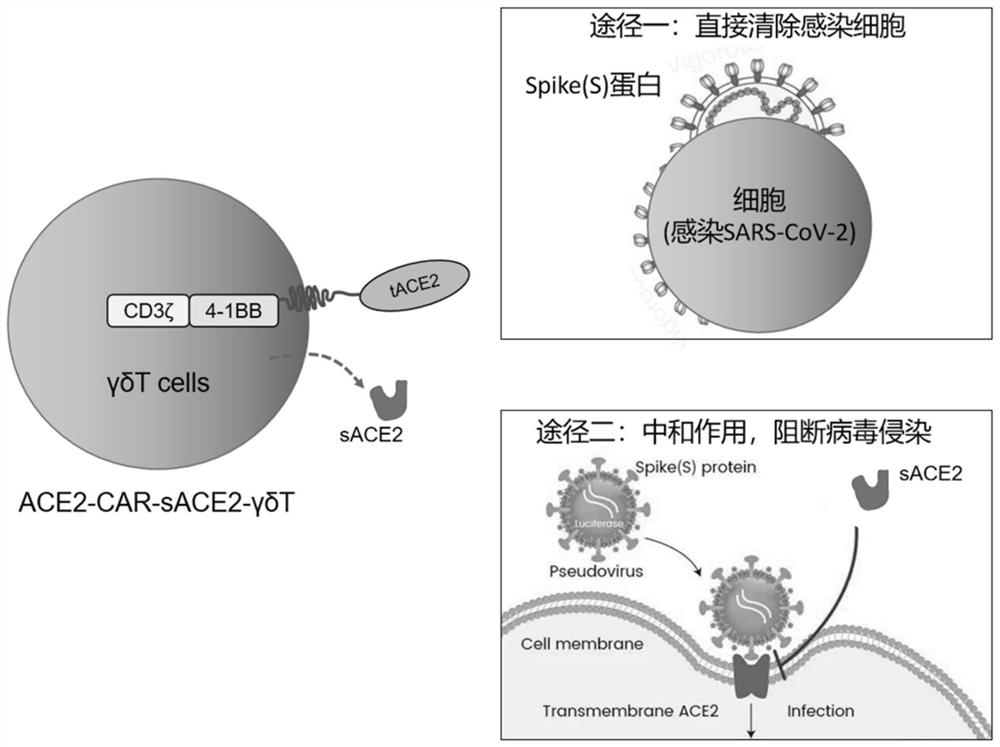
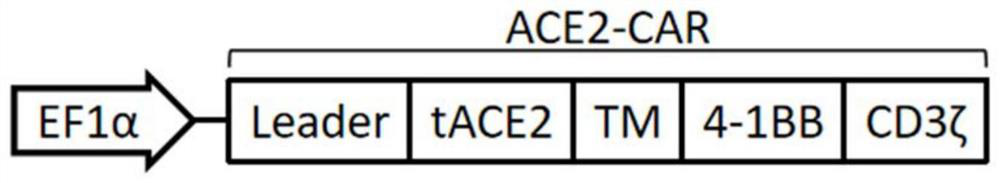
[0083] In this example, a chimeric antigen receptor ACE2-CAR with the 18-615 amino acids of human angiotensin-converting enzyme 2 (ACE2) as the antigen-binding domain was constructed, and on the basis of ACE2-CAR, a human Secreted angiotensin-converting enzyme 2 (human secreted ACE2, sACE2) functional chimeric antigen receptor ACE2-CAR-sACE2, the schematic diagram of the two chimeric antigen receptors is shown in Figure 2A , Figure 2B and image 3 As shown, ACE2-CAR includes a signal peptide (Leader), an antigen-binding domain (tACE2) of ACE2 extracellularly recognizing S protein, a hinge region (Hinge) and a transmembrane region (TM) of CD8α, a costimulatory domain of 4-1BB and CD3ζ signaling domain, ACE2-CAR-sACE2 includes signal peptide (Leader), ACE2 extracellular recognition S protein antigen binding domain (tACE2), CD8α hinge region (Hinge) and transmembrane region (TM), 4-1BB co- Stimulatory domain, CD3ζ signal...
Embodiment 2
[0085] Example 2 lentiviral packaging
[0086] The coding genes of ACE2-CAR and ACE2-CAR-sACE2 were respectively constructed into lentiviral vectors, and the four-plasmid system was used to package the two lentiviral vectors constructed by lentiviruses, and the steps were as follows;
[0087] Mix the helper plasmids gag / pol, Rev and VSV-G with one of the two lentiviral vectors in proportion, with a total mass of 10 μg, add it to a certain volume of serum-free DMEM, mix well and let it stand for 15 minutes; add the above mixture to In a cell culture flask with 293T cells, mix gently and store at 37°C, 5% CO 2 Cultivate in the cell incubator for 6 hours; after 6 hours, replace the fresh medium, continue to cultivate, and add 10mM sodium butyrate solution; after 72 hours, collect the lentivirus culture supernatant for purification and detection.
Embodiment 3
[0088] Example 3 Preparation of CAR-γδT cells
[0089] (1) Separation of PBMCs
[0090] Collect 50mL of peripheral blood; add 15mL of lymphocyte separation medium to two 50mL sterilized centrifuge tubes in the ultra-clean workbench, slowly inject 25-30mL of peripheral blood into the centrifuge tubes containing lymphocyte separation Centrifuge the centrifuge tube at 700g for 20 minutes at room temperature, increase the speed by 1, and decrease the speed by 2. If the blood is stored for more than 2 hours, increase the centrifugation time to 30 minutes;
[0091] After centrifugation, the blood is divided into 4 layers, which are composed of plasma (upper layer), mononuclear cells between plasma and separation liquid (2nd layer), separation liquid (3rd layer) and red blood cells (bottom layer). Collect the mononuclear cells into a new centrifuge tube, add 20mL PBS to dilute the cell suspension, centrifuge at 500g for 10min; remove the supernatant, add 20mL PBS to dilute the cell ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com