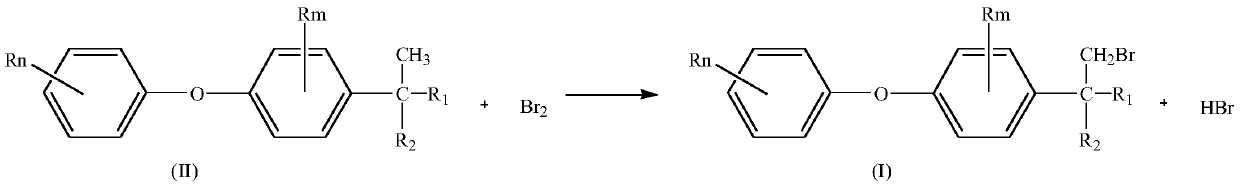
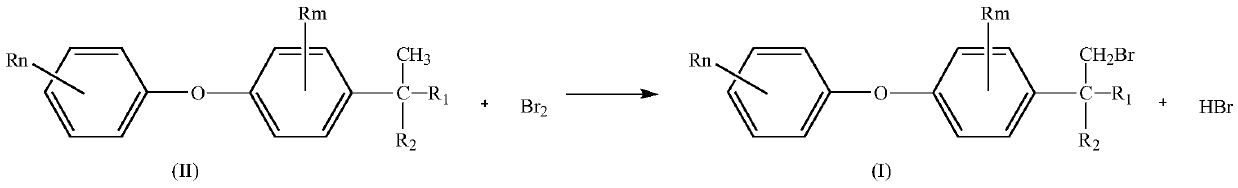
Preparation method of substituted diphenyl ether bromide
A kind of technology of bromide, diphenyl ether
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
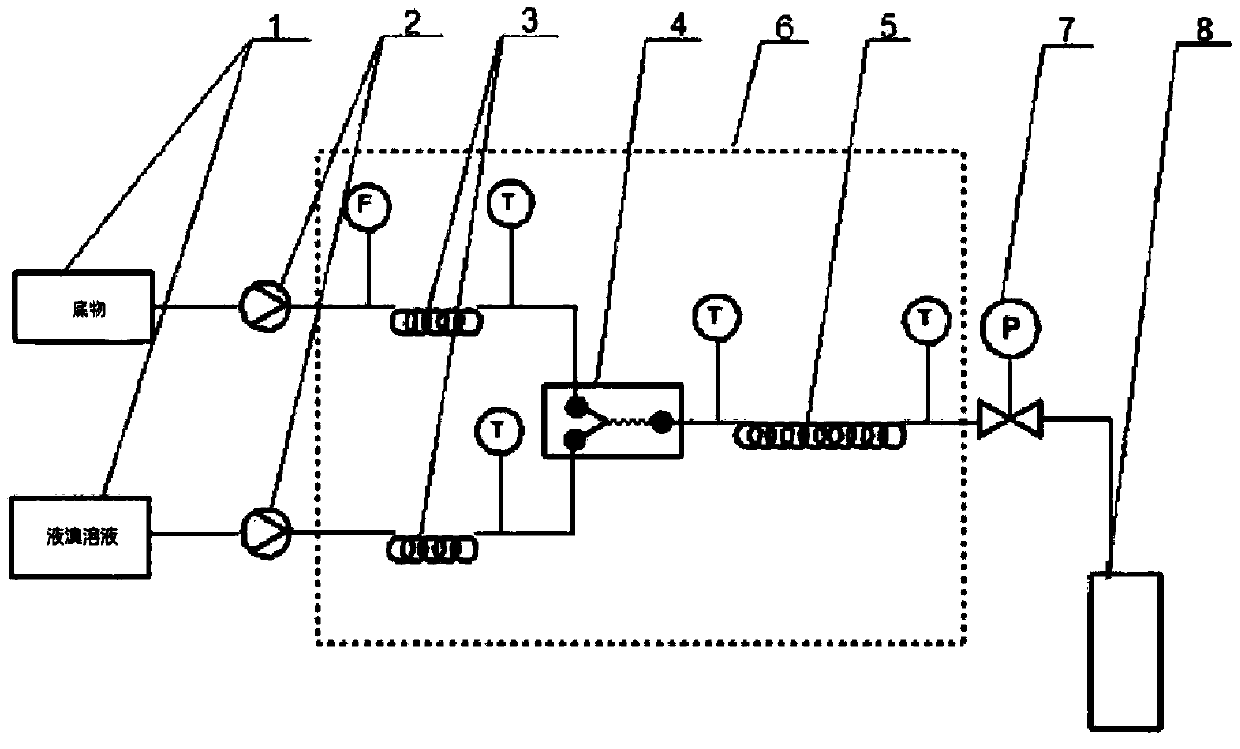
[0044] A kind of preparation method of the bromide of substituted diphenyl ether, concrete steps are as follows:
[0045] 0.070mol of 2-methyl-2-[2-chloro-4-(4-chlorophenoxy)phenyl]-4-methyl-1,3-dioxolane (23.7g) was dissolved in 23.7 g cyclohexane, after mixing, add in the raw material bottle 1, add 11.5g cyclohexane in the 0.072mol liquid bromine (11.5g) and add in the raw material bottle 2 after mixing, open the temperature control system, make the microchannel reactor The temperature of the solution is 30°C. Preheat the cyclohexane solution of the cyclic compound in the raw material bottle 1 to 20-25°C with a flow pump, and enter the microreactor at a flow rate of 1ml / min. At the same time, the bromine solution in the raw material bottle 2 The cyclohexane solution is preheated to 20-25°C in advance, and enters the microreactor at a flow rate of 1.8ml / min. Through the temperature control system, the two react in the microreactor at 25-30°C. Sampling is analyzed by liquid c...
Embodiment 2
[0047] A kind of preparation method of the bromide of substituted diphenyl ether, concrete steps are as follows:
[0048] Dissolve 1mol of 2-methyl-2-[2-chloro-4-(4-chlorophenoxy)phenyl]-4-methyl-1,3-dioxolane (338.05g) in 338.05g In cyclohexane, add in raw material bottle 1 after mixing, add 164.6g cyclohexane in 1.03mol liquid bromine (164.6g) and add in raw material bottle 2 after mixing, open temperature control system, make the microchannel reactor The temperature is 30°C. Preheat the cyclohexane solution of the cyclic compound in the raw material bottle 1 to 20-25°C with a flow pump, and enter the microreactor at a flow rate of 3.5ml / min. At the same time, the bromine solution in the raw material bottle 2 is The cyclohexane solution is preheated to 20-25°C in advance, and enters the microreactor at a flow rate of 1.2ml / min. Through the temperature control system, the two react in the microreactor at 25-30°C. Sampling is analyzed by liquid chromatography. After the react...
Embodiment 3
[0050] A kind of preparation method of the bromide of substituted diphenyl ether, concrete steps are as follows:
[0051] Dissolve 2mol of 2-methyl-2-[2-chloro-4-(4-methylphenoxy)phenyl]-4-n-propyl-1,3-dioxolane (695g) in 700g In cyclohexane, add in raw material bottle 1 after mixing, add 321.6g cyclohexane in 2.01mol liquid bromine (321.6g) and add in raw material bottle 2 after mixing, open temperature control system, make the microchannel reactor The temperature is 30°C. Preheat the cyclohexane solution of the cyclic compound in the raw material bottle 1 to 20-25°C with a flow pump, and enter the microreactor at a flow rate of 4.0ml / min. At the same time, the bromine solution in the raw material bottle 2 is The cyclohexane solution is preheated to 20-25°C in advance, and enters the microreactor at a flow rate of 1.3ml / min. Through the temperature control system, the two react in the microreactor at 25-30°C. Sampling is analyzed by liquid chromatography. After the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com