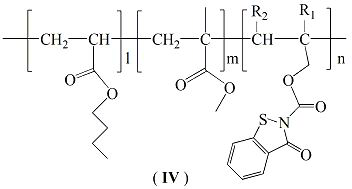
Structure and preparation method of acrylic antifouling resin grafted with benzisothiazolinone formate monomer
An acrylate-based, isothiazoline technology used in antifouling/underwater coatings, anti-corrosion coatings, biocide-containing paints, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
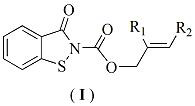
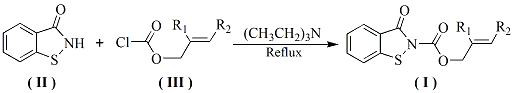
[0027] Preparation of benzo[d]isothiazolin-3-one-2-carboxylate allyl alcohol ester monomer: in a 100 ml three-necked flask equipped with a stirring device, a condenser tube and a thermometer, add 1.51g (0.01 mol ) benzo [d] Isothiazolin-3-one, 1.21 g (0.01mol) allyl alcohol chloroformate and a catalytic amount of triethylamine were added to a 100 mL three-necked flask, and 25 ml of toluene was added. After the dissolution was complete, the reaction was stirred at room temperature for 2.0 Hours, TLC tracking reaction to complete. The reactant was cooled to room temperature, filtered with suction, and the filtrate was collected, washed with water, neutralized, dried, concentrated and evaporated to dryness, and the residue was separated and purified to obtain a light yellow solid with a yield of 84.5%.
Embodiment 2
[0029] The preparation of benzo [d] isothiazolin-3-one-2-formic acid-2-methallyl alcohol ester monomer: in the 100 ml there-necked flask equipped with stirring device, condenser and thermometer, add 1.51g ( Add 0.01 mol) benzo[d]isothiazolin-3-one, 1.35g (0.01 mol) 2-methylallyl chloroformate and catalytic amount of triethylamine into a 100 mL three-necked flask, add 25ml of toluene , after the dissolution was complete, the reaction was stirred at 50° C. for 2.0 hours, followed by TLC until the reaction was complete. The reactant was cooled to room temperature, filtered with suction, and the filtrate was collected, washed with water, neutralized, dried, concentrated and evaporated to dryness, and the residue was separated and purified to obtain a white solid with a yield of 83%.
Embodiment 3
[0031] Preparation of benzo[d]isothiazolin-3-one-2-formic acid-2-ethyl allyl alcohol ester monomer: in a 100 ml three-necked flask equipped with stirring device, condenser and thermometer, add 1.51g ( 0.01 mol) benzo[d]isothiazolin-3-one, 1.48g (0.01 mol) 2-ethyl allyl chloroformate and a catalytic amount of triethylamine were added to a 100 mL three-necked flask, and 25ml of toluene was added , after the dissolution was complete, the reaction was stirred at 50° C. for 2.0 hours, followed by TLC until the reaction was complete. The reactant was cooled to room temperature, filtered with suction, and the filtrate was collected, washed with water, neutralized, dried, concentrated and evaporated to dryness, and the residue was separated and purified to obtain a white solid with a yield of 79.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com