Potassium manganate potassium ion battery positive electrode material
A battery positive electrode, potassium ion technology, applied in battery electrodes, positive electrodes, secondary batteries, etc., can solve problems such as inability to meet energy demand, and achieve the effects of low preparation cost, easy operation, and pure material phase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
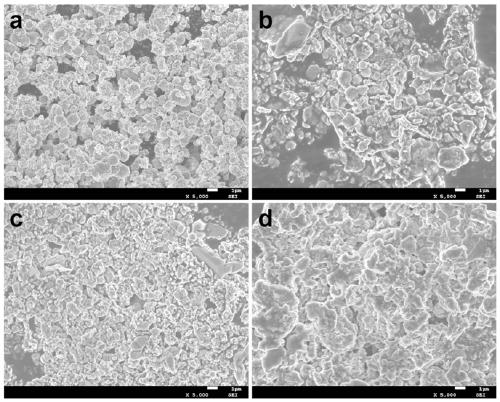
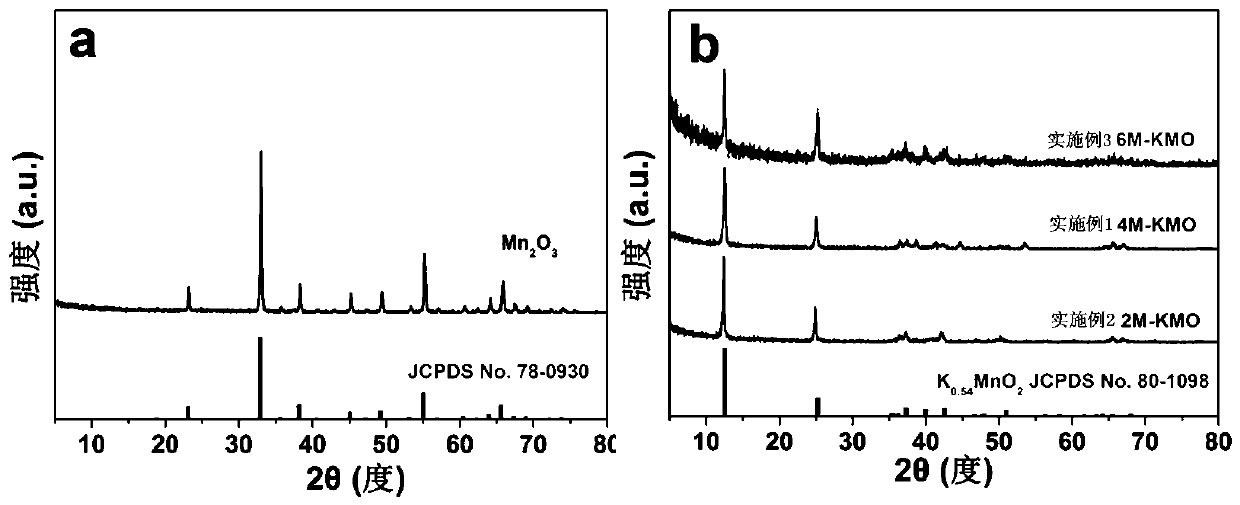
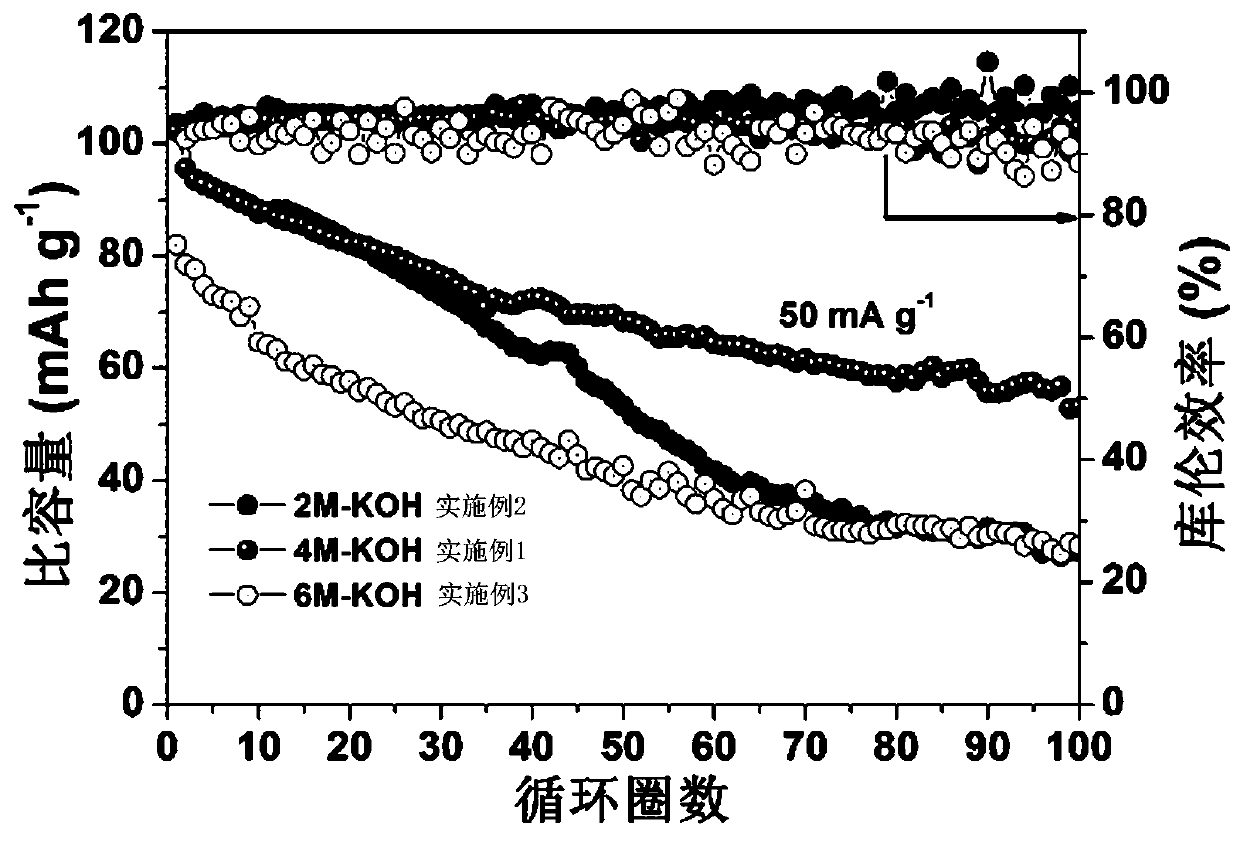
[0016] Put 0.025 mol of manganese chloride tetrahydrate powder and 0.025 mol of sodium carbonate powder in two beakers and add 25 mL of deionized water to each, and continue to stir until they are completely dissolved. The manganese chloride tetrahydrate was added dropwise to the sodium carbonate solution under a stirring speed of 200 r / min, and stirring was continued for 5 minutes after the addition, and then the precipitate was filtered, washed and dried, and placed in the air at 600 rpm. Annealed at ℃ for 4 h to obtain the precursor Mn 2 O 3 Microspheres. 0.004mol precursor Mn 2 O 3 The microspheres were dispersed in a KOH solution with a concentration of 4 M and reacted hydrothermally at 200 ℃ for 12 hours. The precipitate was directly dried and then placed in an air atmosphere at 700 ℃ and annealed for 3 hours to obtain K. x MnO 2 Cathode material for potassium ion battery. In order to understand the morphology of the sample, 2 O 3 And K x MnO 2 The samples were tested by ...
Embodiment 2
[0018] Put 0.025 mol of manganese chloride tetrahydrate powder and 0.025 mol of sodium carbonate powder in two beakers and add 25 mL of deionized water to each, and continue to stir until they are completely dissolved. The manganese chloride tetrahydrate was added dropwise to the sodium carbonate solution under a stirring speed of 200 r / min, and stirring was continued for 5 minutes after the addition, and then the precipitate was filtered, washed and dried, and placed in the air at 600 rpm. Annealed at ℃ for 2 h to obtain the precursor Mn 2 O 3 Microspheres. 0.004mol precursor Mn 2 O 3 The microspheres were dispersed in a KOH solution with a concentration of 2 M and reacted hydrothermally at 200 ℃ for 12 h. The precipitate was directly dried and then placed in an air atmosphere at 700 ℃ for annealing treatment for 3 h to obtain K x MnO 2 Cathode material for potassium ion battery. Such as figure 2 As shown in b, for the samples obtained after 2M KOH treatment, there are K at 1...
Embodiment 3
[0020] Put 0.025 mol of manganese chloride tetrahydrate powder and 0.025 mol of sodium carbonate powder in two beakers and add 25 mL of deionized water to each, and continue to stir until they are completely dissolved. The manganese chloride tetrahydrate was added dropwise to the sodium carbonate solution under a stirring speed of 200 r / min, and stirring was continued for 5 minutes after the addition, and then the precipitate was filtered, washed and dried, and placed in the air at 600 rpm. Annealed at ℃ for 6 h to obtain the precursor Mn 2 O 3 Microspheres. 0.004mol precursor Mn 2 O 3 The microspheres were dispersed in a KOH solution with a concentration of 6 M and reacted hydrothermally at 200°C for 12 hours. The precipitate was directly dried and then placed in an air atmosphere at 700°C and annealed for 3 hours to obtain K. x MnO 2 Cathode material for potassium ion battery. For the samples obtained after 6M KOH treatment, there are K at 12.54°, 25.24°, 35.36°, 36.21°, 39.6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com