Thienopyrrole derivative as well as preparation method and application thereof
A technology of pyrrole derivatives and thiophene, which is applied in the field of thienopyrrole derivatives and their preparation, can solve the problems of weak inhibition of colon cancer proliferation, unsatisfactory half-inhibition rate, and many impurities in the final product, and achieve inhibition of colon cancer proliferation, The preparation method is simple and feasible, and the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
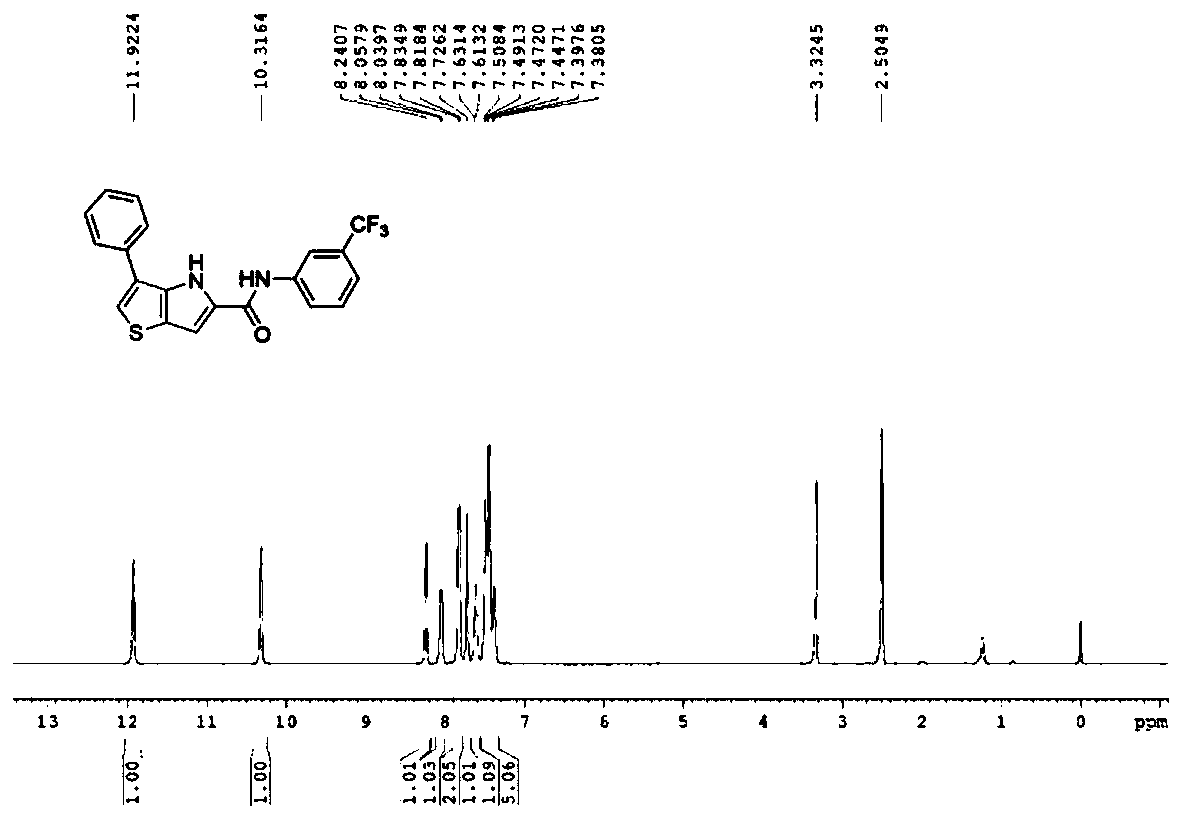
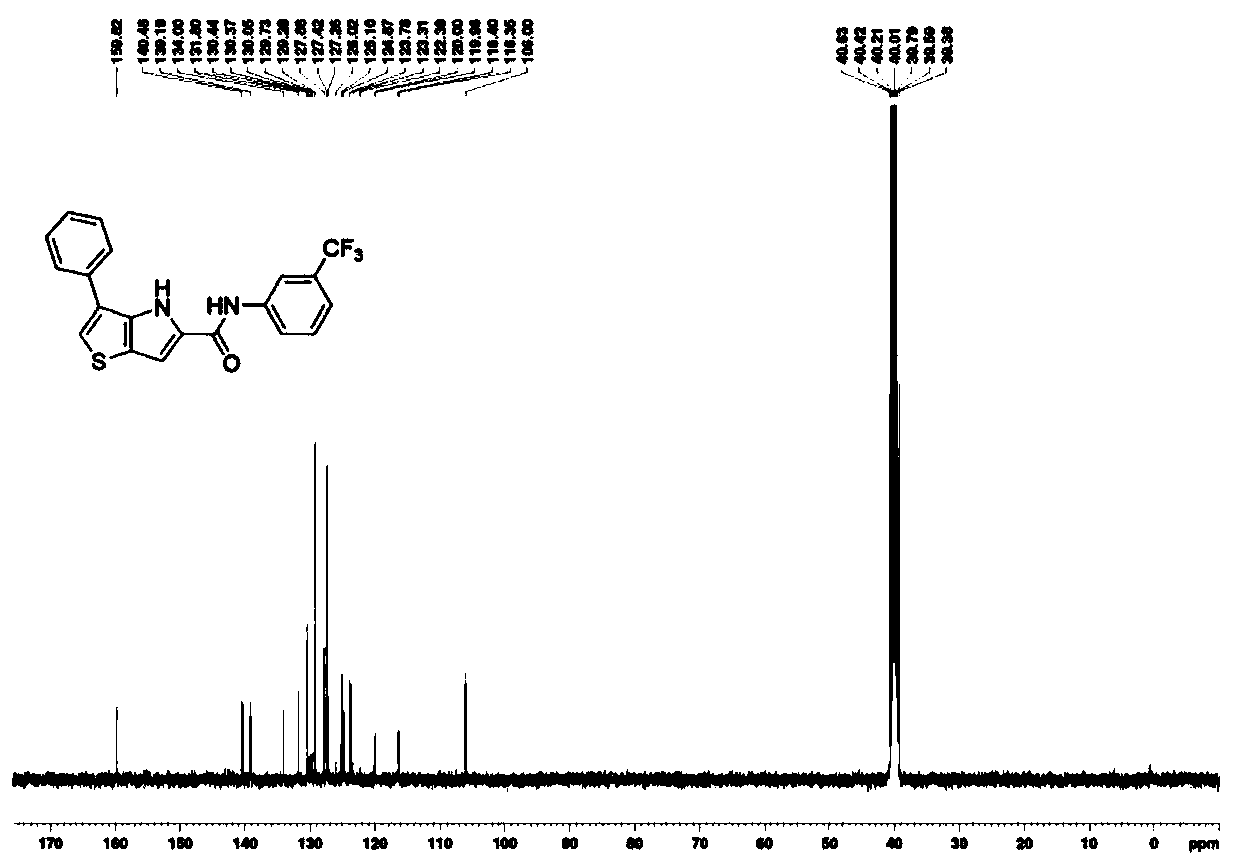
Examples
Embodiment 1
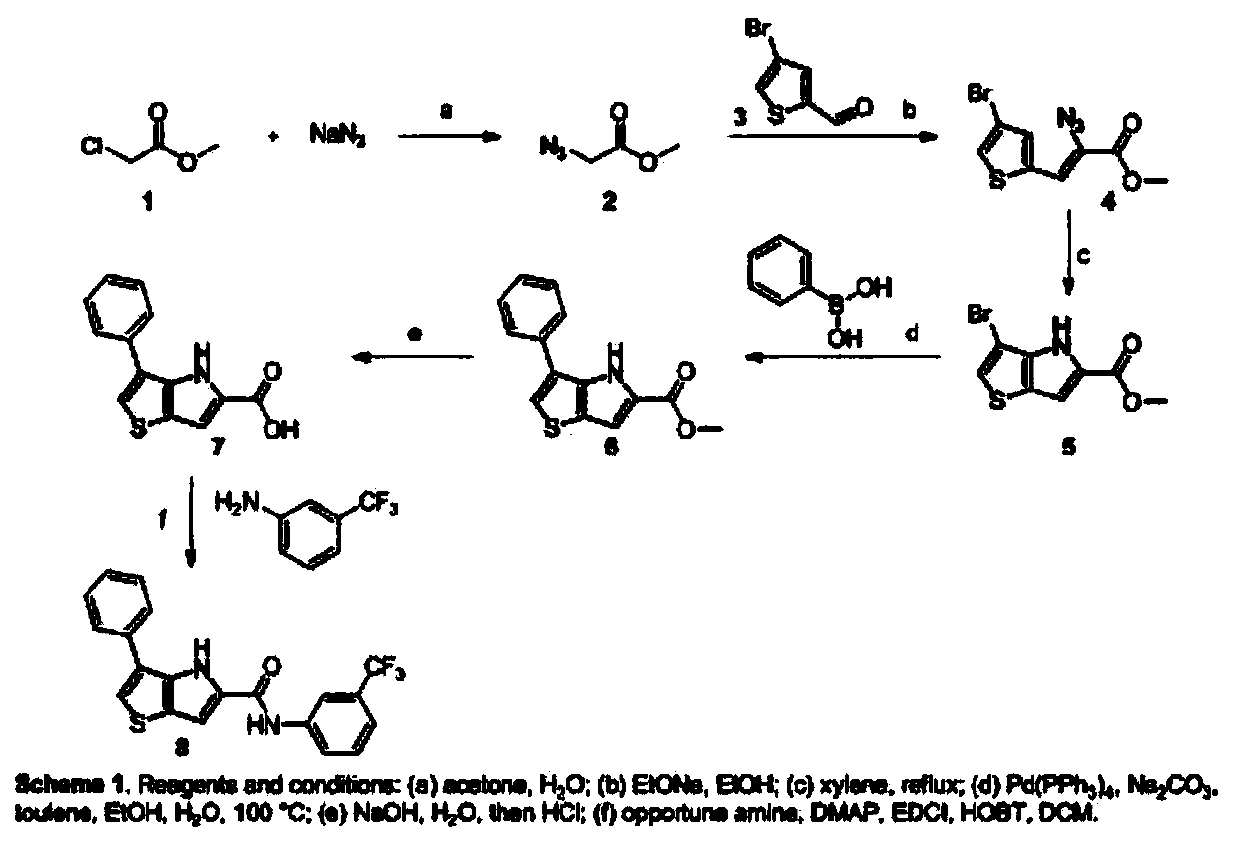
[0048] A preparation method of thienopyrrole derivatives, which is prepared according to the following steps:
[0049] (1) Synthesis of 2-methyl azidocarboxylate:
[0050] Add sodium azide into the acetone / water solution of methyl chloroacetate under stirring at room temperature, then heat to 55-65°C for 4 hours, then place the reaction solution in a rotary evaporator, set the vacuum to -0.05--0.085MPa Rotary evaporation at a temperature of 45-55°C, the obtained residue was extracted with ethyl acetate, and then washed with an equal volume of saturated sodium bicarbonate solution and saturated saline solution respectively, after washing, anhydrous sodium sulfate was added to dry for 13 hours, filtered and dried After concentration, light yellow oily methyl 2-azidocarboxylate is obtained; the mol ratio of sodium azide to methyl chloroacetate is 2:1, and the mass volume ratio of sodium azide to methyl chloroacetate in acetone / water solution is 13: 200, the volume ratio of aceto...
Embodiment 2
[0063] A preparation method of thienopyrrole derivatives, which is prepared according to the following steps:
[0064] (1) Synthesis of 2-methyl azidocarboxylate:
[0065] Add sodium azide into the acetone / water solution of methyl chloroacetate under stirring at room temperature, then heat to 55~65°C for 3 hours, then put the reaction solution in a rotary evaporator, set the vacuum degree to -0.05MPa~-0.08 MPa, the temperature is 45 ℃ for rotary evaporation, the obtained residue is extracted with ethyl acetate, and then washed with an equal volume of saturated sodium bicarbonate solution and saturated saline solution respectively. After washing, anhydrous sodium sulfate is added to dry for 12 hours, and filtered Yellow oily 2-methyl azidocarboxylate; The mol ratio of sodium azide to methyl chloroacetate is 2:1, the mass volume ratio of sodium azide to the acetone / water solution of methyl chloroacetate is 13:200, acetone / The volume ratio of water is 2:1; the mass volume ratio...
Embodiment 3
[0078] A preparation method of thienopyrrole derivatives, which is prepared according to the following steps:
[0079] (1) Synthesis of 2-methyl azidocarboxylate:
[0080] Add sodium azide into the acetone / water solution of methyl chloroacetate under stirring at room temperature, then heat to 55-65°C for 5 hours, then place the reaction solution in a rotary evaporator, and set the vacuum degree to -0.05MPa~-0.08 MPa, the temperature is 55°C for rotary evaporation, the obtained residue is extracted with ethyl acetate, and then washed with an equal volume of saturated sodium bicarbonate solution and saturated saline solution respectively. After washing, anhydrous sodium sulfate is added to dry for 15 hours, and filtered Yellow oily 2-methyl azidocarboxylate; The mol ratio of sodium azide to methyl chloroacetate is 2:1, the mass volume ratio of sodium azide to the acetone / water solution of methyl chloroacetate is 13:200, acetone / The volume ratio of water is 2:1; the mass volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com