Application of canagliflozin in treatment of pancreatic cancer
A technology for pancreatic cancer and drugs, which is applied in the field of preparation of drugs for treating pancreatic cancer, can solve problems such as adverse reactions and allergic reactions, achieve the effects of inhibiting glycolysis, inhibiting apoptosis, and good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
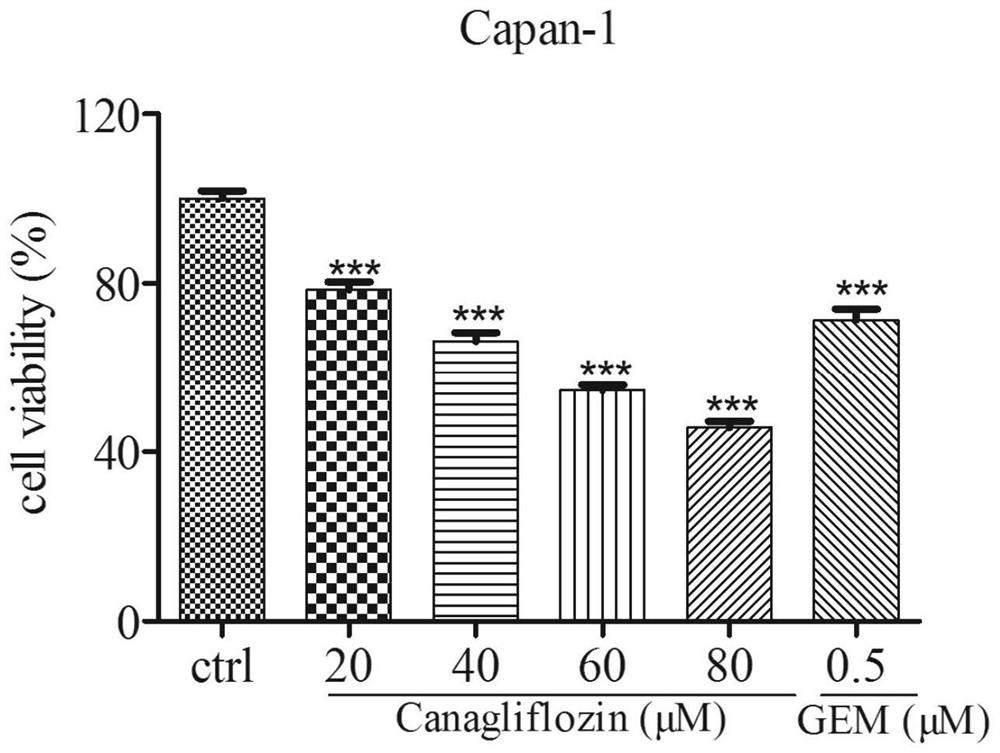
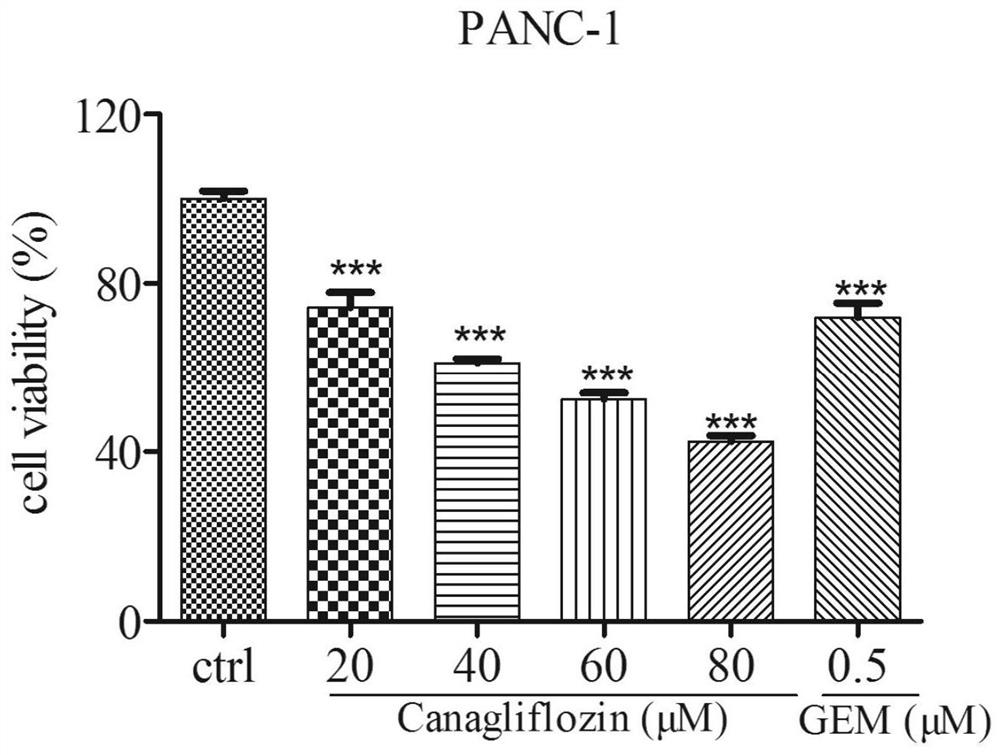
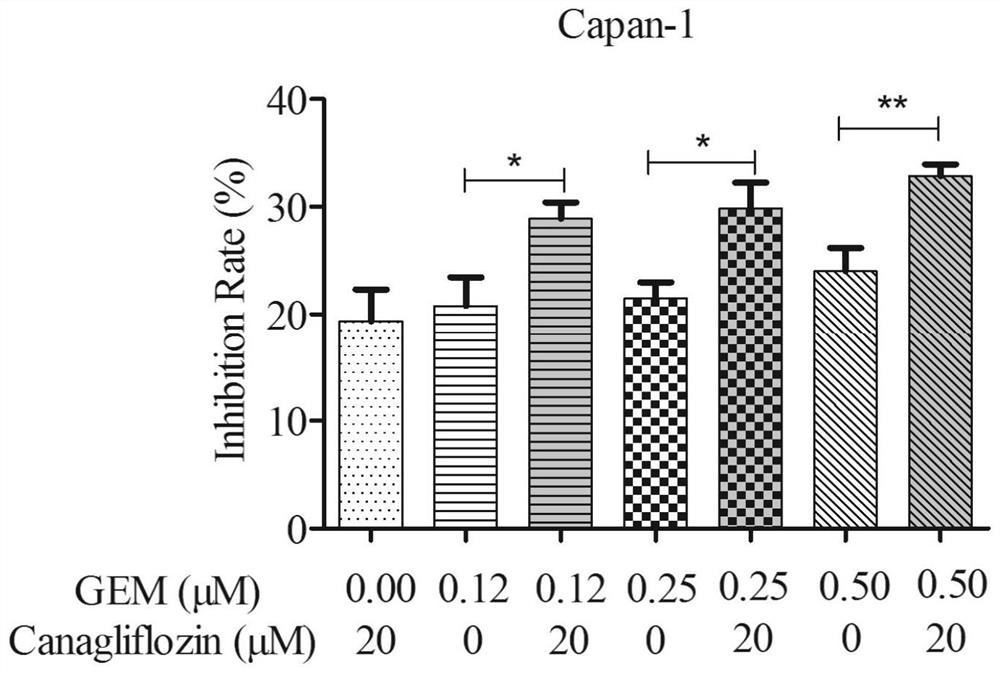
[0038] Antitumor activity of canagliflozin against pancreatic cancer cells:
[0039] 1. Solution preparation
[0040] (1) Canagliflozin solution: weigh canagliflozin raw material, dissolve in 1mL DMSO, and prepare 80mmol / L mother solution, pass through a 0.22μm microporous membrane, filter and sterilize, and then dispense into multiple 200μL EP tubes were stored in a -20°C refrigerator for later use.
[0041] (2) DMEM medium: take DMEM medium freeze-dried powder, dissolve in 1L double distilled water, stir magnetically, add about 1.6g sodium bicarbonate, adjust the pH of the solution to 7.2, pass through a 0.22μm filter membrane in a sterile environment Sterilize by filtration and store at 4°C until use. When culturing the cells, 10% Gibco fetal bovine serum and 100 U / mL penicillin-streptomycin were added to the culture medium.
[0042] (3) Trypsin: weigh 4.0g of NaCl, 0.1g of KCl, NaCl 2 HPO 4 12H 2 O 1.45g, KH 2 PO 4 Dissolve 0.1g in 400mL of double-distilled water,...
Embodiment 2
[0072] Antitumor activity of canagliflozin on pancreatic cancer tumor-bearing mice:
[0073] 1. Establishment of pancreatic cancer PANC-1 tumor-bearing mouse xenograft model
[0074] Balb / c male mice were kept in the animal experiment center of China Pharmaceutical University with a temperature of 22±1°C and 12 hours of light per day, providing them with sufficient food and water, and the animal experiment operations followed the animal ethics of China Pharmaceutical University committee. Take PANC-1 cells in good growth state and in the logarithmic growth phase, digest the cells, wash with normal saline three times, wash off the residual medium on the cell surface, add normal saline, and suspend evenly. By counting, adjust the PANC-1 cell density to 6 × 10 6 individual / mL. Inject 100 μL of cell suspension under the armpit of each nude mouse, and after one week, the tumor grows to about 80-100 mm 3 At the same time, the Balb / c male nude mice were randomly divided into 6 gr...
Embodiment 3
[0083] Canagliflozin inhibits glycolysis in pancreatic cancer cells:
[0084] 1. Glucose uptake experiment
[0085] Glucose content was detected by a glucose kit. Experimental principle: Glucose in the sample can be oxidized by glucose oxidase into gluconic acid and hydrogen peroxide, and hydrogen peroxide can couple the reduced 4-aminoantipyrine to phenol under the catalysis of peroxidase Condensation into quinone compounds that can be measured by spectrophotometer. Place the culture plate with the cells in an incubator for culture. After it adheres to the wall, add canagliflozin to treat it. Take out the cell supernatant at 6h, 12h and 24h after administration, add the working solution and mix thoroughly. Put the sample tube into the spectrophotometer whose OD value is adjusted to 505nm wavelength, measure the absorbance value of each sample tube, and finally record the data and process the result.
[0086] Depend on Figure 12 and Figure 13 As shown, the glucose uptak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com