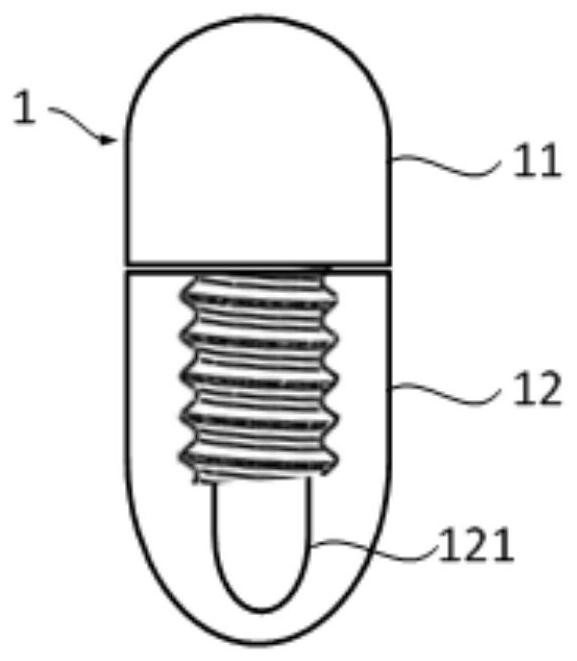
A capsule for monitoring the pH value of gastric acid by nuclear magnetic resonance imaging and its preparation method
A nuclear magnetic resonance imaging and capsule technology, which is applied in the fields of capsule delivery, application, and pharmaceutical formulation, etc., can solve the problem of easy interference of amplification ability and pH value response ability, reduce the amplification effect of HSA-Mn system, and non-mono-responsiveness of pH and other problems, to achieve the effect of non-destructive monitoring of gastric acid pH, low production cost, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0111] The third aspect of the present invention provides a method for preparing the capsule shell, the method comprising the following steps:
[0112] 1) preparing a gel solution, heating the gel solution until clear and transparent;
[0113] 2) pouring the gel solution in step 1) into the capsule cavity of the mould, inserting the capsule core into the capsule cavity,
[0114] and / or,
[0115] The gel solution in step 1) is poured into the cap cavity of the mould;
[0116] 3) Leave it for a certain period of time until the gel is formed, then remove the gel from the mold to obtain the capsule shell;
[0117] In a preferred embodiment, the concentration of the gel solution in step 1) is 1-5% (mass percentage), for example, it may be 3%.
[0118] In step 1), the gel is heated until it is clear and transparent without air bubbles, which can ensure the uniformity of the prepared capsule shell.
[0119] In step 2), the heated gel solution needs to be quickly poured into the...
Embodiment 1
[0136] Example 1 HSA-Mn solution NMR signal amplification and pH responsiveness
[0137] In order to explore the nuclear magnetic signal amplification effect of HSA-Mn solution under specific detection conditions (magnetic field strength, temperature), different concentrations of HSA-Mn solutions were prepared, in which manganese ions were derived from manganese chloride (manufacturer: Aladdin Biochemical Technology Co., Ltd., Product number: M299158), the original concentration is 0.05M, that is, 2750mg / L, diluted with deionized water into two parts, A solution, the concentration is 100mg / L, and B solution, the concentration is 1000mg / L. HSA comes from Beijing Baishayi Technology Co., Ltd., product number BS027. The specific method of preparation is as follows:
[0138] 1) Mix manganese chloride and MOPS buffer according to Table 1. Each group prepares two solutions with different pH (pH=1 and pH=7), and the different pH is realized by the pH value of the MOPS buffer soluti...
Embodiment 2
[0151] The preparation of embodiment 2 hydrogel capsules
[0152] 1. Preparation of hydrogel capsule shell:
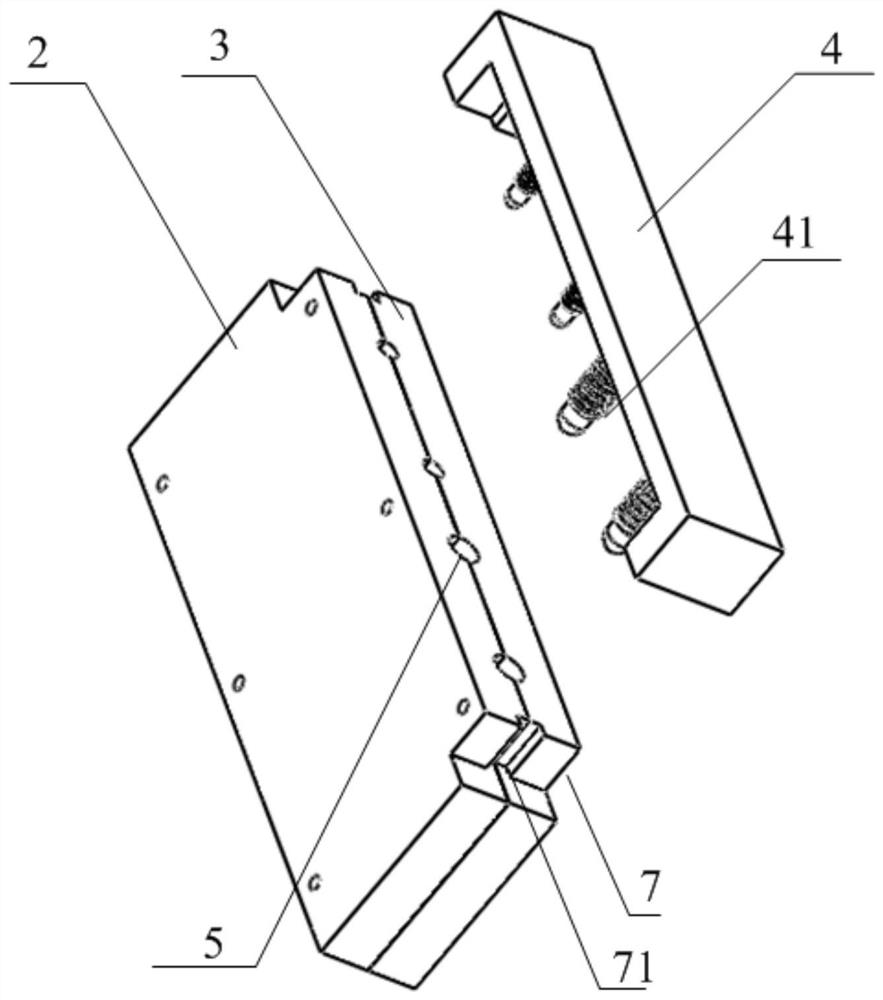
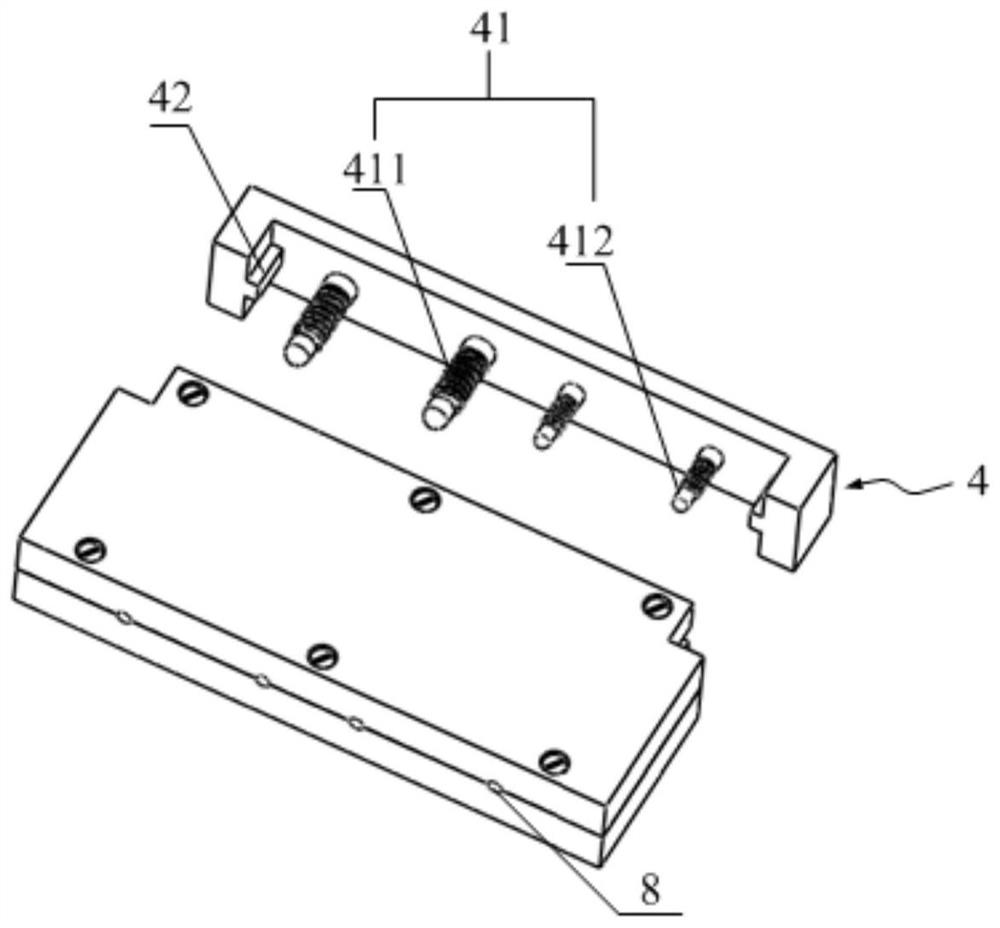
[0153] Combination mold: first put together the first template and the second template, screw 6 screws in, and lock the joint surface, such as figure 2 As shown, a complete capsule cavity is formed;
[0154] Gel pouring: heating 3% (mass percentage) agarose gel to 90 degrees Celsius to make the gel clear and transparent without bubbles, and quickly pour it into the capsule cavity;
[0155] Molding: press the third template figure 2 Insert it into the cavity of the capsule body as shown, place the mold at room temperature (25 degrees Celsius) for 5 minutes, and then transfer to an environment of 4 degrees Celsius for another 5 minutes;
[0156]Demoulding: Unscrew 6 screws, immerse the mold and the coagulated gel in water, slowly and carefully uncover the first template and the second template, and after the gel is filled and wet, remove the gel from the capsule of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com