Synthesis method of rimsulfuron
A synthesis method and technology of rimsulfuron-methyl, applied in the field of herbicide synthesis, can solve the problems of low yield, existence of impurities, low yield and the like, and achieve the effects of high purity, few impurities and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
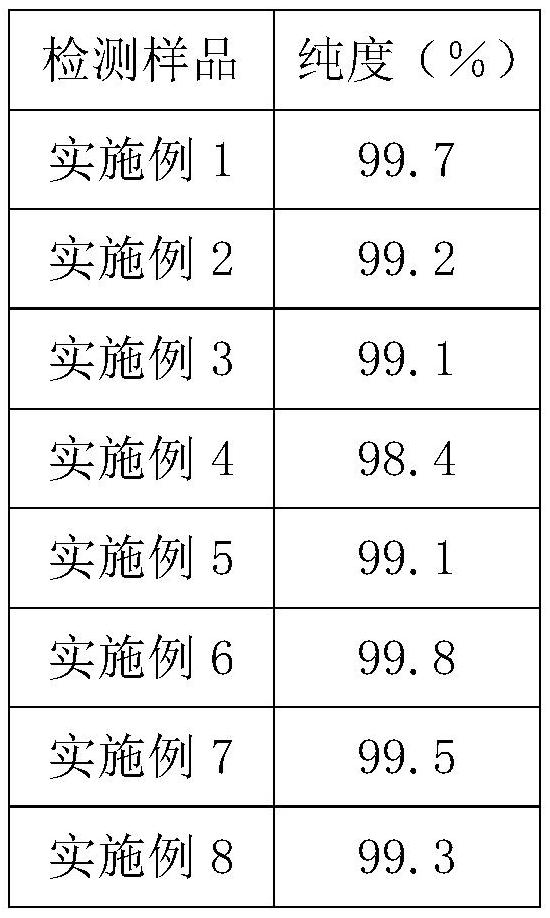
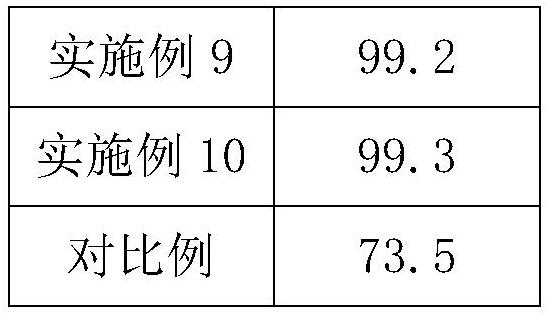
Examples
Embodiment 1
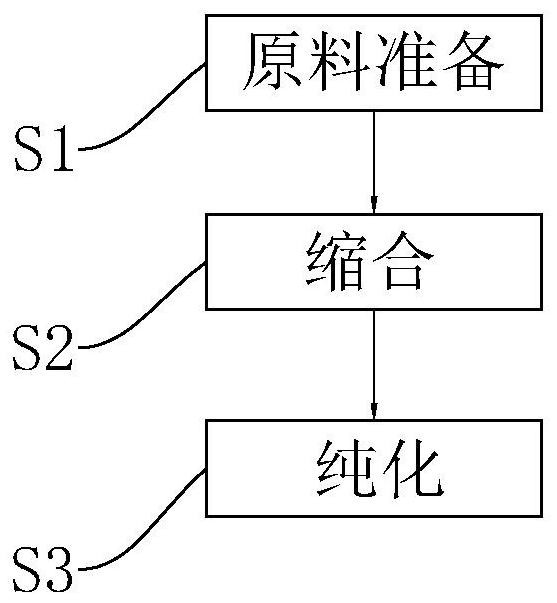
[0031] refer to figure 1 , a synthetic method of rimsulfuron-methyl, comprising the steps of:
[0032] S1: Raw material preparation. Prepare 3-ethanesulfonyl-2-ethanesulfonamidopyridine and 4,6-dimethoxypyrimidine-2-carbamate phenyl ester.
[0033] Preparation of 3-ethanesulfonyl-2-ethanesulfonamidopyridine:
[0034] First add 20kg of water, 10kg of hydrochloric acid and 54kg of dichloromethane into the first container, then add 18kg of 3-ethanesulfonyl-2-mercaptopyridine, start stirring, and at the same time reduce the temperature in the first container to 4°C and keep warm 3min. Then start dripping sodium hypochlorite, the dripping amount of sodium hypochlorite is 170kg, and the dropping temperature is 13 ℃, after dropping, it is incubated for 15min, then drips ammoniacal liquor in the organic phase, and the dripping amount of ammoniacal liquor is 36kg, and the sampling analysis ammonia transformation rate is After the concentration is above 85%, hydrochloric acid is add...
Embodiment 2
[0046] The difference from Example 1 is that S3: purification. Including the following steps:
[0047] A. Weigh 20kg of the rimsulfuron-methyl crude product obtained in S2, add it to the first solvent, heat it to 70°C under stirring, and keep it warm for 3h; the first solvent is 40kg, and then heat the liquid system to 30°C for 10h. The first solvent is chloroform.
[0048] B. Add a second solvent to the liquid treated in step A, then cool the liquid system to 5°C, then filter the precipitate, and take the filter cake for later use; the amount of the second solvent added is 20kg; the second solvent is octane.
[0049] C. Add the filter cake obtained in step B into the third solvent, stir and beat, and keep the beating temperature at 25° C. for 4 hours; the amount of the third solvent added is 16 kg; the third solvent is anisole.
[0050] D. Filtering and drying the substance treated in step C to obtain the original drug of rimsulfuron-methyl.
Embodiment 3
[0052] The difference from Example 1 is that S3: purification. Including the following steps:
[0053] A. Weigh 20kg of the rimsulfuron-methyl crude product obtained in S2, add it to the first solvent, heat it to 70°C under stirring, and keep it warm for 3h; the first solvent is 40kg, and then heat the liquid system to 30°C for 10h. The first solvent is carbon tetrachloride.
[0054] B. Add a second solvent to the liquid treated in step A, then cool the liquid system to 5°C, then filter the precipitate, and take the filter cake for later use; the amount of the second solvent added is 20kg; the second solvent is toluene.
[0055] C. Add the filter cake obtained in step B into the third solvent, stir and beat, and keep the beating temperature at 25°C for 4 hours; the amount of the third solvent added is 16kg; the third solvent is methyl tert-butyl ether .
[0056] D. Filtering and drying the substance treated in step C to obtain the original drug of rimsulfuron-methyl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com