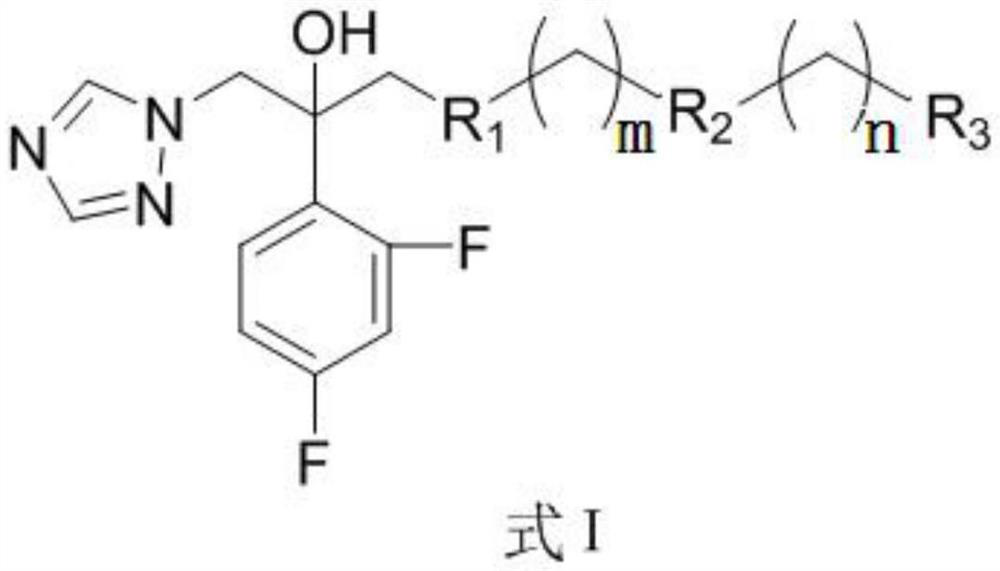
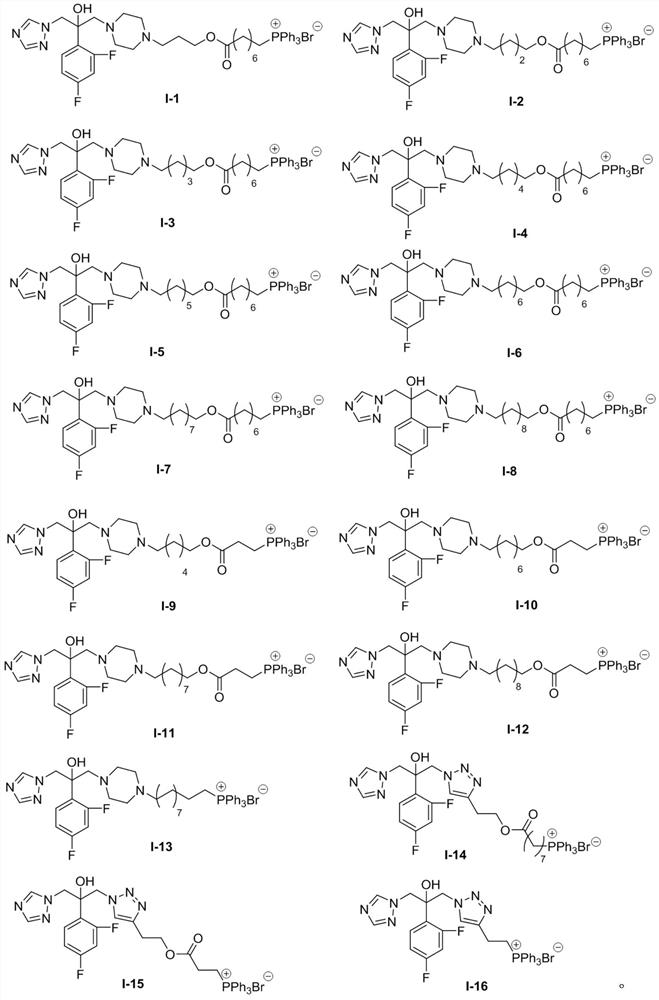
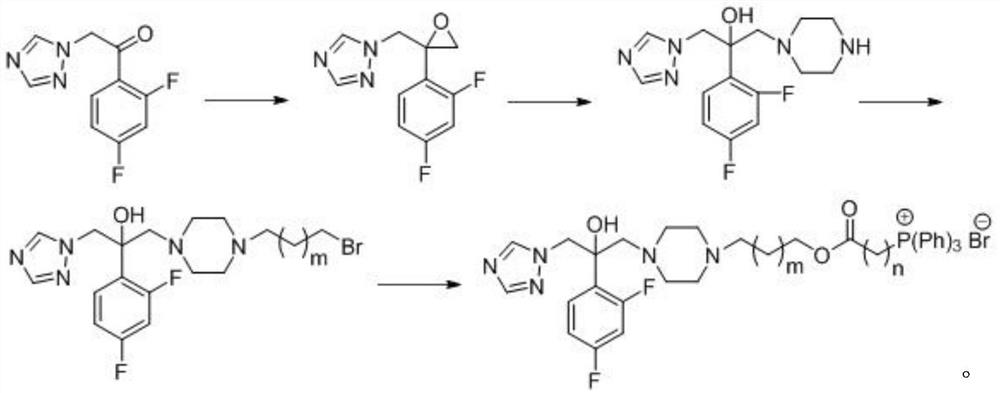
Triazole compounds, preparation method and application of triazole compounds in antifungal drugs
An antifungal drug, triazole technology, applied in the direction of antifungal agents, chemical instruments and methods, compounds of elements of Group 5/15 of the periodic table, etc. To achieve the effect of strong druggability, overcoming drug resistance problems and improving activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of compound I-1
[0032] Step 1: Preparation of Amphiphilic Cationic Compounds
[0033] Weighed triphenylphosphine (5g) and dissolved it in toluene (30ml), added 8-bromooctanoic acid (3g), and refluxed at 110°C for 12h. After the reaction, the liquid in the reaction bottle was directly poured out, the solid in the bottle was washed 3 times with ethyl acetate, and after the ethyl acetate was evaporated to dryness, a light yellow oily substance 1 was obtained.
[0034]
[0035] Weighed triphenylphosphine (5g) and dissolved it in toluene (30ml), added 3-bromopropionic acid (2g), and refluxed at 110°C for 12h. After the reaction, the reaction liquid was directly poured out, washed 3 times with ethyl acetate, and after the ethyl acetate was evaporated to dryness, a light yellow oily substance 2 was obtained.
[0036]
[0037] Step 2: Preparation of Compound 4
[0038] Weigh 2,4-difluoro-2-(1H-1,2,4-triazol-1-yl)acetophenone (5g) into a ...
Embodiment 2
[0046] Embodiment 2: the preparation of compound 1-2
[0047] Step 1: Preparation of compound 5-2
[0048] To a DMF solution of compound 1 (50 mg), add 1,4-dibromobutane (122.6 μL) and K 2 CO 3 (28.55 mg) and the reaction was monitored by TLC. The reaction mixture was stirred at room temperature for 24 hours, the reaction mixture was diluted with dichloromethane and washed with water, the organic phases were combined, washed with brine, dried over anhydrous magnesium sulfate, and the solvent was spin-dried in vacuo to obtain the crude product, which was purified by flash chromatography to obtain White solid compound 5-2. 1 H NMR (500MHz, Methanol-d 4 )δ7.92–7.87(m,3H),7.81(t,J=6.2Hz,4H),7.81–7.73(m,9H),4.10(t,J=6.3Hz,2H),3.44–3.37(m ,2H),2.30(t,J=7.3Hz,2H),1.77(dt,J=12.9,6.5Hz,2H),1.68(dq,J=16.0,8.2Hz,2H),1.57(dp,J= 14.9,7.3Hz,4H),1.41–1.25(m,6H).
[0049]
[0050] Step 2: Preparation of Compound I-2
[0051] In the DMF solution of compound 4 (25mg), add compound 5-...
Embodiment 3
[0053] Embodiment 3: the preparation of compound 1-3
[0054] Step 1: Preparation of compound 5-3
[0055] To a DMF solution of compound 1 (50 mg), add 1,5-dibromopentane (143 μL) and K 2 CO 3 (28.55 mg) and the reaction was monitored by TLC. The reaction mixture was stirred at room temperature for 24 hours, the reaction mixture was diluted with dichloromethane and washed with water, the organic phases were combined, washed with brine, dried over anhydrous magnesium sulfate, and the solvent was spin-dried in vacuo to obtain the crude product, which was purified by flash chromatography to obtain White solid compound 5-3. 1 H NMR (500MHz, Chloroform-d) δ7.87 (dd, J = 12.4, 7.7Hz, 6H), 7.78 (t, J = 7.1Hz, 3H), 7.70 (dt, J = 10.5, 5.2Hz, 6H) ,4.04(t,J=6.5Hz,2H),3.92–3.83(m,2H),2.24(t,J=7.4Hz,2H),1.64(dt,J=13.3,7.3Hz,8H),1.54( dd,J=12.6,5.9Hz,2H),1.52–1.45(m,2H),1.34–1.20(m,6H).
[0056]
[0057] Step 2: Preparation of Compound I-3
[0058] In the DMF solution of compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com