Preparation method of supported CuNi bimetallic catalyst and application of supported CuNi bimetallic catalyst in reduction reaction
A bimetallic catalyst, supported technology, applied in the direction of catalyst activation/preparation, preparation of organic compounds, metal/metal oxide/metal hydroxide catalyst, etc., can solve high cost, unfavorable production promotion and application, catalytic activity High abundance, good electrical conductivity, and stable chemical structure can be achieved without high problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of Supported Inexpensive Bimetallic Cu 1 Ni 1 / rGO catalyst, comprising the following steps:
[0056] (1) Accurately weigh 10 mg copper acetylacetonate (Cu(acac) 2 ), 9.8mg nickel acetylacetonate (Ni(acac) 2 ), 6mg ferric chloride (FeCl 3 ·6H 2 O), 52.8mg ascorbic acid (vitamin C, C 6 h 8 o 6 ) and 5mL oleylamine (OAm) were added to a 100mL round-bottomed flask A, and the round-bottomed flask A was placed in an ultrasonic instrument for ultrasonication for 1 h until the mixture was evenly mixed with oleylamine, and it was a transparent reddish-brown solution, until no solid matter appeared;
[0057] (2) Add pre-prepared 50mg graphene oxide (GO) and 10mL deionized water into a 100mL round-bottomed flask B, ultrasonicate it for 2h until graphene oxide is completely dissolved in deionized water, and the solution is muddy yellow;
[0058] (3) Add the graphene oxide aqueous solution in round-bottom flask B dropwise to round-bottom flask A at a rate of abo...
Embodiment 2
[0063] In the present invention, the prepared catalyst is characterized by transmission electron microscope (TEM) characterization, high-resolution transmission electron microscope (HRTEM) characterization, and X-ray powder diffraction (XRD) characterization methods, and the results are as follows:
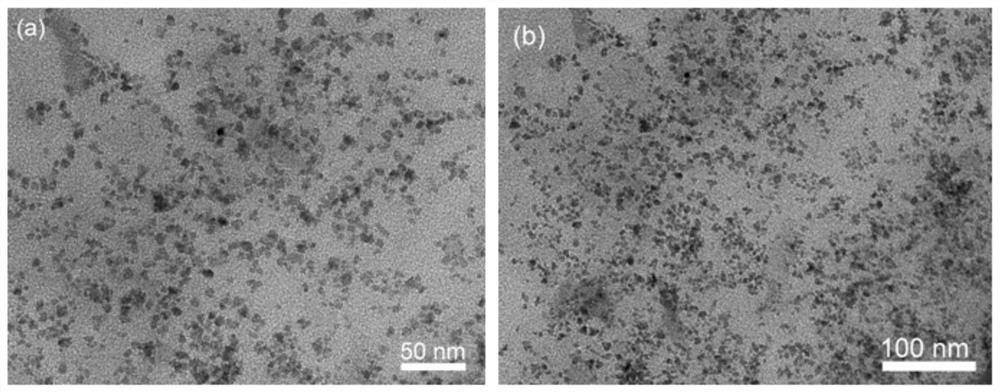
[0064] 1. Transmission electron microscopy (TEM) characterization
[0065] Cu by transmission electron microscopy (TEM) 1 Ni 1 / rGO catalysts were characterized, as shown in the attached figure 1 As shown in (a) and (b), the catalyst is uniformly dispersed and uniform in size, without obvious agglomeration phenomenon, and its particle size is about 5nm.
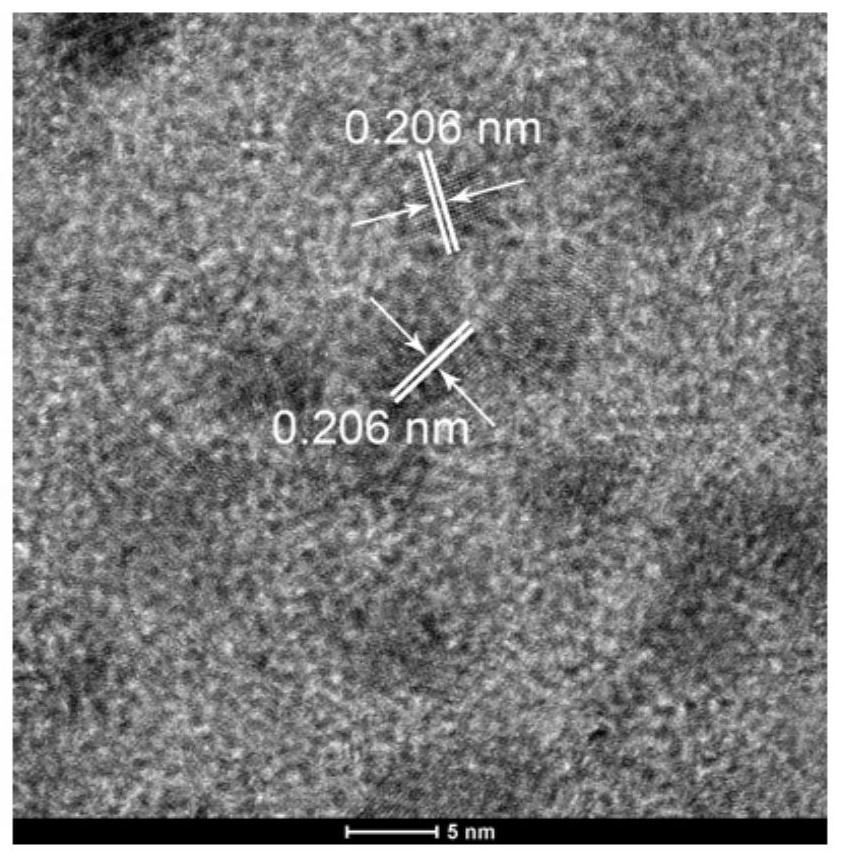
[0066] 2. Characterization by High Resolution Transmission Electron Microscopy (HRTEM)
[0067] Cu by high-resolution transmission electron microscopy (HRTEM) 1 Ni 1 / rGO catalysts were characterized, as shown in the attached figure 2 As shown, its lattice spacing is 0.206nm.
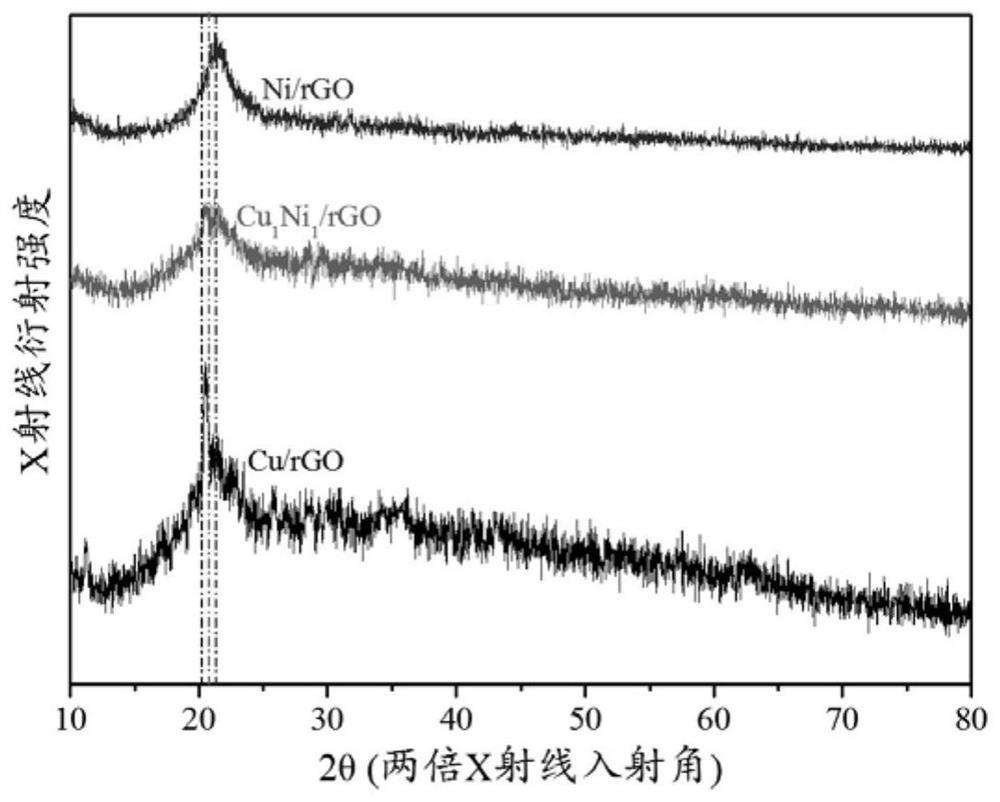
[0068] 3. X-ray powder diffraction (XRD) cha...
Embodiment 3
[0071] About Cu 1 Ni 1 / rGO catalyzes the hydrogenation reduction reaction of nitrobenzene and its derivatives. The inventors optimized the reaction conditions, expanded the reaction substrate, and investigated the recyclability of the catalyst. The details are as follows:
[0072] 1. Cu 1 Ni 1 Optimization of Reaction Conditions for Hydrogenation Reduction of Nitrobenzene Catalyzed by / rGO
[0073] At present, in the field of catalytic chemistry, precious metals that are expensive and difficult to obtain are mainly used, such as palladium (Pd), gold (Au), ruthenium (Ru), rhodium (Rh), iridium (Ir), platinum (Pt), etc. catalyst. In the present invention, metal Cu and Ni that are cheap and easy to obtain are used to load on reduced graphene oxide (Cu 1 Ni 1 / rGO) as a catalyst is of great significance in catalytic chemistry, so Cu 1 Ni 1 / rGO has shown promising application prospects in the hydrogenation reduction catalysis industry.
[0074] Cu 1 Ni 1 / rGO is used t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com