Method for producing high-purity anhydrous aluminum chloride from aluminum hydroxide
An anhydrous aluminum chloride and aluminum hydroxide technology, applied in the directions of aluminum chloride, aluminum halide, energy input, etc., can solve the problems of complicated operation process, not considering heat removal, reducing equipment capacity, etc., to achieve enhanced transfer and Reaction process, avoid rapid corrosion, reduce the effect of process energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
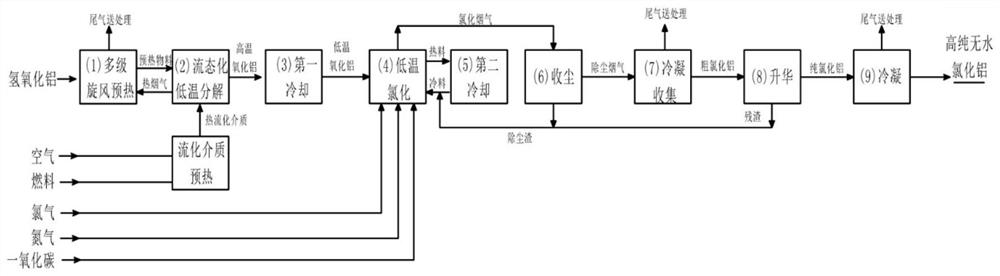
[0048] figure 1 It is a schematic flow chart of the method for producing high-purity anhydrous aluminum chloride from aluminum hydroxide described in the present invention. combine figure 1 , a method for producing high-purity anhydrous aluminum chloride from aluminum hydroxide used in this embodiment, the method includes the following steps: multi-stage cyclone preheating step 1; fluidized decomposition step 2; first cooling Process 3; low-temperature chlorination process 4; second cooling process 5; chlorination flue gas dust collection process 6; condensation collection process 7; sublimation process 8; condensation process 9, specifically as follows:
[0049] 1) Aluminum hydroxide is sent to the multi-stage cyclone preheating process 1, and the hot flue gas obtained by fluidized decomposition is used to preheat the aluminum hydroxide material;
[0050] 2) sending the preheated material obtained in the multi-stage cyclone preheating process 1 into the fluidized decomposit...
Embodiment 2
[0059] The present embodiment adopts the method for producing high-purity anhydrous aluminum chloride by aluminum hydroxide described in embodiment 1. The multi-stage cyclone preheating process 1 uses a 3-stage cyclone preheating decomposer, and the preheated raw materials enter the fluidized decomposition process 2, and use a bubbling fluidized bed decomposer to completely decompose into a high-temperature decomposer at 350°C. Alumina powder; vertical baffles are set inside the fluidized bed, and the residence time is 0.1min. The high-temperature alumina powder obtained by decomposition enters the first cooling process 3 and is cooled to 250°C. The low-temperature alumina powder obtained after cooling enters Low-temperature chlorination step 4, chlorine gas and carbon monoxide are passed into the fluidized bed reactor at a molar ratio of 1:0.9 to cause chlorination of alumina at 600°C; part of the unreacted material is cooled to 50°C through the second cooling step 5 After th...
Embodiment 3
[0061] The present embodiment adopts the method for producing high-purity anhydrous aluminum chloride by aluminum hydroxide described in embodiment 1. The multi-stage cyclone preheating process 1 uses a 4-stage cyclone preheating decomposer, and the preheated raw materials enter the fluidized decomposition process 2, and use a bubbling fluidized bed decomposer to completely decompose into high temperature Alumina powder; vertical baffles are set inside the fluidized bed, and the residence time is 20 minutes. The high-temperature alumina powder obtained by decomposition enters the first cooling process 3 and is cooled to 280°C. After cooling, the low-temperature alumina powder obtained enters the low-temperature Chlorination process 4, chlorine gas, carbon monoxide and nitrogen gas are passed into the fluidized bed reactor at a molar ratio of 1:2:1, so that alumina can undergo chlorination reaction at 700°C; some unreacted materials are cooled by the second cooling process 5 Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com