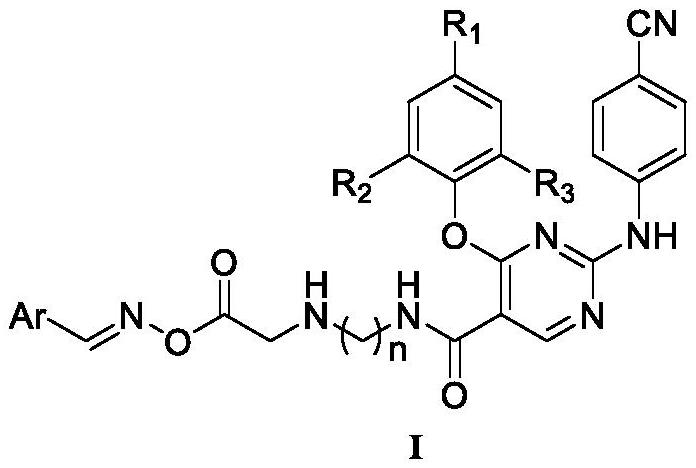
Oxime group-containing diarylpyrimidine HIV-1 reverse transcriptase inhibitor, and preparation method and application thereof
A technology of reverse transcriptase inhibition and arylpyrimidines, which is applied in the field of medicine, can solve the problems of restricting wide application, cross-drug resistance, low water solubility, etc., and achieve the effect of high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: N-(2-(2-(aminooxy)acetamido)ethyl)-4-(4-cyano-2,6-dimethylphenoxy)-2-((4- Preparation of cyanophenyl)amino)pyrimidine-5-carboxamide (A1).
[0041]
[0042] Dissolve ethyl 2,4-dichloro-5-pyrimidinecarboxylate (0.25g, 1.13mmol) and 4-hydroxy-3,5-dimethylbenzonitrile (0.20g, 1.36mmol) in 30mL DMF, add K 2 CO 3 (0.19g, 1.36mmol), stirred at room temperature for 6h, and detected the reaction by TLC. After the reaction, extract with dichloromethane (10mL×3), combine the organic layers, wash with saturated brine (30mL) once, anhydrous Na 2 SO 4 Dry for 5 hours, filter, mix the sample, separate by column, and recrystallize from ethyl acetate / petroleum ether to obtain white solid I-2.
[0043] Palladium acetate (0.028g, 0.125mmol) and 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (0.073g, 0.13mmol) were dissolved in dioxane (15mL), stirred at room temperature After activation for 15 min, I-2 (1.0 g, 3.0 mmol) and cesium carbonate (1.2 g, 3.68 mmol) were added ...
Embodiment 2
[0054] Embodiment 2: the preparation of target compound
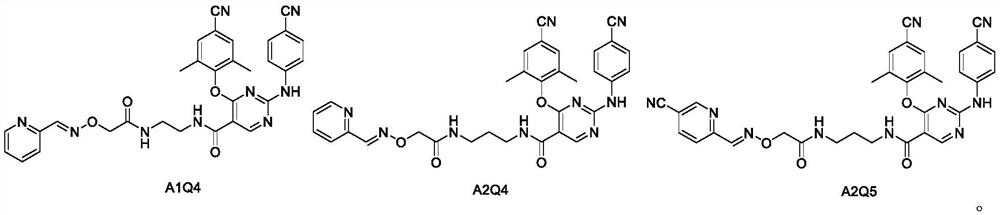
[0055] Dissolve dominant hydroxylamine fragment A1 (0.1g, 0.20mmol), pyridine-2-carbaldehyde (22.8μL, 0.24mmol) in ethanol (10mL), add AcOH (13.7μL, 0.24mmol), reflux at 60°C for 4h, and detect by TLC reaction. After the reaction was complete, the sample was mixed directly, and the head compound A1Q4 was obtained by silica gel column separation.
[0056]
[0057] The product is a white solid, yield: 72%, melting point: 235-237°C.
[0058] 1 H NMR (400MHz, DMSO-d 6 ):δ10.54(s,1H),8.92(s,1H),8.60(d,J=2.8Hz,1H,C 6 -pyrimidine-H),8.30(s,1H),8.24(s,1H),8.08(s,1H),7.80(s,3H),7.72(d,J=7.6Hz,0H),7.47–7.41( m,5H),4.56(s,2H),3.46–3.45(m,2H),3.41–3.34(m,2H),2.14(s,6H,2CH 3 ). 13 C NMR (100MHz, DMSO-d 6):δ169.27,165.22,162.88,162.44,159.81,153.80,151.48,150.92,150.16,144.13,137.38,133.33,133.05,125.18,121.11,119.64,119.06,119.02,109.24,106.06,104.01,73.48,38.83,16.30. HRMS m / z C 31 h 27 N 9 o 4 :calcd 589.22,found 5...
Embodiment 3
[0067] Embodiment 3: In vitro anti-HIV activity test experiment of the target compound
[0068] Test principle:
[0069] The anti-HIV activity of compounds was screened in vitro by MTT method. The full name of MTT is bromide-3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium nitrogen (trade name: thiazolium blue), which can be used to detect the survival and growth of cells. The detection principle is:
[0070] MTT can be combined with succinate dehydrogenase in living cells to be reduced to water-insoluble blue-purple crystalline formazan and deposited in cells, while dead cells do not have this function. Dimethyl sulfoxide can dissolve formazan in cells, and its absorbance (A) value at 590 nm can indirectly reflect the number of living cells by using a microplate reader. Within a certain cell number range, the amount of MTT crystal formation is proportional to the cell number.
[0071] Because the MT-4 cell that HIV infects can take place lesion within a certain period...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com