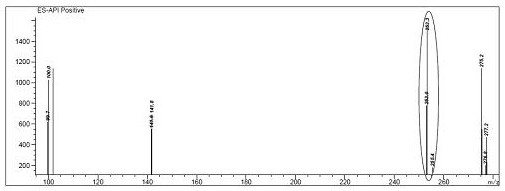
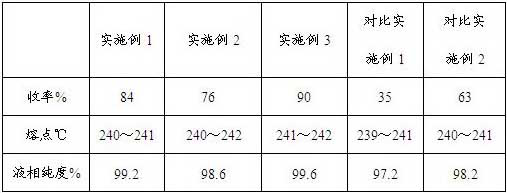
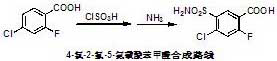
Synthetic method of Azosemide intermediate
A synthesis method and technology of azosemide are applied in the preparation of sulfonic acid, the preparation of sulfonic acid amides, organic chemistry, etc., and can solve the problem of reducing the yield and purity of 4-chloro-2-fluoro-5-sulfamoylbenzoic acid, affecting the Product purity and yield, easy hydrolysis of sulfonyl chloride, etc., to achieve the effect of simple and convenient production process, excellent yield, and inhibition of isomer impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 120 milliliters of chlorosulfonic acid and 25 grams of sodium chloride to the reaction flask, raise the temperature to 100°C, add 50 grams of 4-chloro-2-fluorobenzoic acid in batches, react at 100°C for another 1 hour, and then raise the temperature to 120 ℃ reaction for 5 hours, to achieve staged heating to promote the reaction, thereby effectively inhibiting the generation of isomer impurities. After the reaction, the reaction solution was cooled to room temperature, slowly poured onto crushed ice, stirred sufficiently, then filtered with suction, and the obtained filter cake was washed with ice water.
[0036] Cool 500ml of isopropanol to 0°C, feed 25g of ammonia gas, control the temperature below 10°C, add the above-mentioned filter cake in batches, naturally raise the temperature and stir overnight; then concentrate to remove isopropanol, add 200ml of water Stir for 1 hour, acidify with hydrochloric acid until the pH is 2-3, cool and crystallize, filter with su...
Embodiment 2
[0038] Add 120 ml of chlorosulfonic acid and 30 g of ammonium chloride to the reaction flask, add 50 g of 4-chloro-2-fluorobenzoic acid in batches, then raise the temperature to 100°C for 1 hour, then raise the temperature to 160°C for 1 hour , to achieve staged heating to promote the reaction, thereby effectively inhibiting the generation of isomer impurities. After the reaction, cool the reaction liquid with ice salt, control the temperature below 20°C, slowly add 500 ml of cold water dropwise to extract the reaction, filter with suction, and wash the obtained filter cake with ice water.
[0039] Add 120 grams of ammonium carbonate to the obtained filter cake, grind continuously for 2 hours, leave it overnight, add 200 milliliters of water and stir, acidify with hydrochloric acid until the pH is 2 to 3, cool and crystallize, suction filter, wash with water, and the volume ratio of the solid used is The 10:1 ethanol-water mixture was recrystallized twice, and dried to obtain ...
Embodiment 3
[0041] Add 150 ml of chlorosulfonic acid and 20 g of lithium chloride to the reaction flask, raise the temperature to 80°C, add 50 g of 4-chloro-2-fluorobenzoic acid in batches, raise the temperature to 100°C for 1 hour, and then raise the temperature to 120°C After 5 hours of reaction, staged heating is realized to promote the reaction, thereby effectively suppressing the generation of isomer impurities. After the reaction, the reaction solution was cooled to room temperature, slowly poured onto crushed ice, stirred fully, then filtered with suction, and the obtained filter cake was washed with ice water.
[0042]Grind the filter cake and 85 grams of ammonium chloride evenly, gradually add solid sodium carbonate in small batches for full grinding, and wait until the system does not emit carbon dioxide gas and the system is alkaline, and continue the grinding reaction for 1 hour. Add 250 ml of water and stir, acidify with hydrochloric acid until the pH value is 2-3, cool and c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com