Preparation method of hydrogel for adsorbing anionic dye and metal ions by carbon dioxide response expansion
A technology of anionic dyes and metal ions, applied in chemical instruments and methods, adsorption of water/sewage treatment, alkali metal compounds, etc., to achieve the effects of avoiding dangerous operations, good cyclic expansion and contraction performance, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
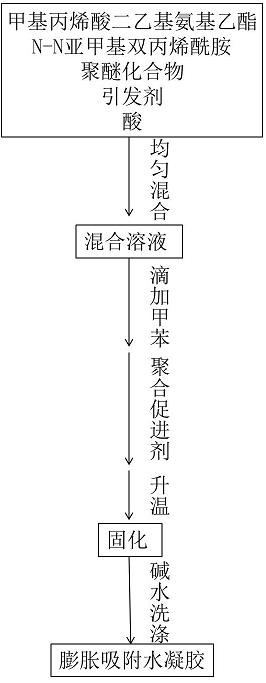
[0028] Add 5% mass fraction of diethylaminoethyl methacrylate, 0.5% mass fraction of N,N-methylenebisacrylamide, 1% mass fraction of Pluronic F127, and 5% mass fraction in the reaction vessel Hydrochloric acid, ammonium persulfate with a mass fraction of 0.2%, water with a mass fraction of 18.25%, after uniform stirring, drop in toluene with a mass fraction of 70% and mix evenly, then add tetramethylethylenediamine with a mass fraction of 0.05%, at 40°C After reacting for 18 hours, after polymerization, it was neutralized to neutrality with 0.5% by mass sodium hydroxide aqueous solution to obtain carbon dioxide responsive expansion to adsorb anionic dyes and metal ion hydrogels.
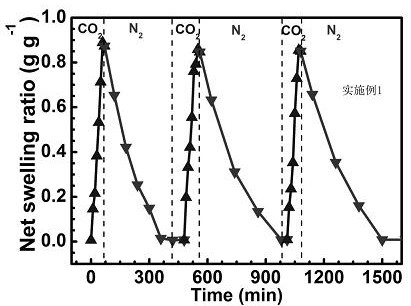
[0029] The carbon dioxide and nitrogen response of the carbon dioxide response swelling adsorption anion dye and metal ion hydrogel obtained in this example can be cyclically expanded and contracted, such as figure 2 shown.
Embodiment 2
[0031] Add 5% mass fraction of diethylaminoethyl methacrylate, 0.5% mass fraction of N,N-methylenebisacrylamide, 1% mass fraction of Pluronic F127, and 5% mass fraction in the reaction vessel Hydrochloric acid, ammonium persulfate with a mass fraction of 0.2%, water with a mass fraction of 18.25%, after uniform stirring, drop in toluene with a mass fraction of 70% and mix evenly, then add tetramethylethylenediamine with a mass fraction of 0.05%, at 45°C After reacting for 18 hours, after polymerization, it was neutralized to neutrality with 0.5% by mass sodium hydroxide aqueous solution to obtain carbon dioxide responsive expansion to adsorb anionic dyes and metal ion hydrogels.
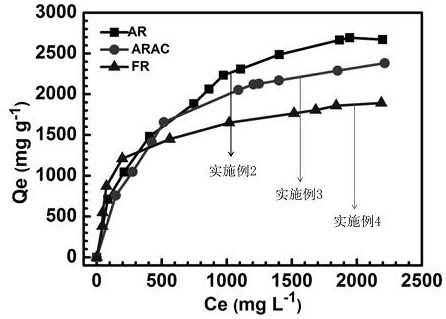
[0032] This embodiment is to anionic dye alizarin red adsorption performance such as image 3 As shown, the adsorption capacity reached 2696 mg g -1 .
Embodiment 3
[0034] Add 10% mass fraction of diethylaminoethyl methacrylate, 0.1% mass fraction of N,N-methylenebisacrylamide, 0.5% mass fraction of Pluronic F127, and 1% mass fraction in the reaction vessel Hydrochloric acid, ammonium persulfate with a mass fraction of 0.1%, water with a mass fraction of 13.29%, after uniform stirring, drop in toluene with a mass fraction of 75%, mix evenly, add tetramethylethylenediamine with a mass fraction of 0.01%, and at 50°C After reacting for 18 hours, after polymerization, it was neutralized to neutrality with 0.5% by mass sodium hydroxide aqueous solution to obtain carbon dioxide responsive expansion to adsorb anionic dyes and metal ion hydrogels.
[0035] The present embodiment is to the anionic dye allura red adsorption performance such as image 3 As shown, the adsorption capacity reached 2381 mg g -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com