Application of compound in preparation of medicine for inhibiting bacterial quorum sensing system
A quorum sensing system and antibacterial technology, applied in the field of medicine, can solve the problems of changing the physiological and biochemical characteristics of bacteria, reducing the effect of antibiotics, and reducing the sensitivity of antibiotics to pathogenic bacteria, so as to reduce the pathogenicity and virulence of bacteria, Does not inhibit growth, slows down the effect of bacterial multidrug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
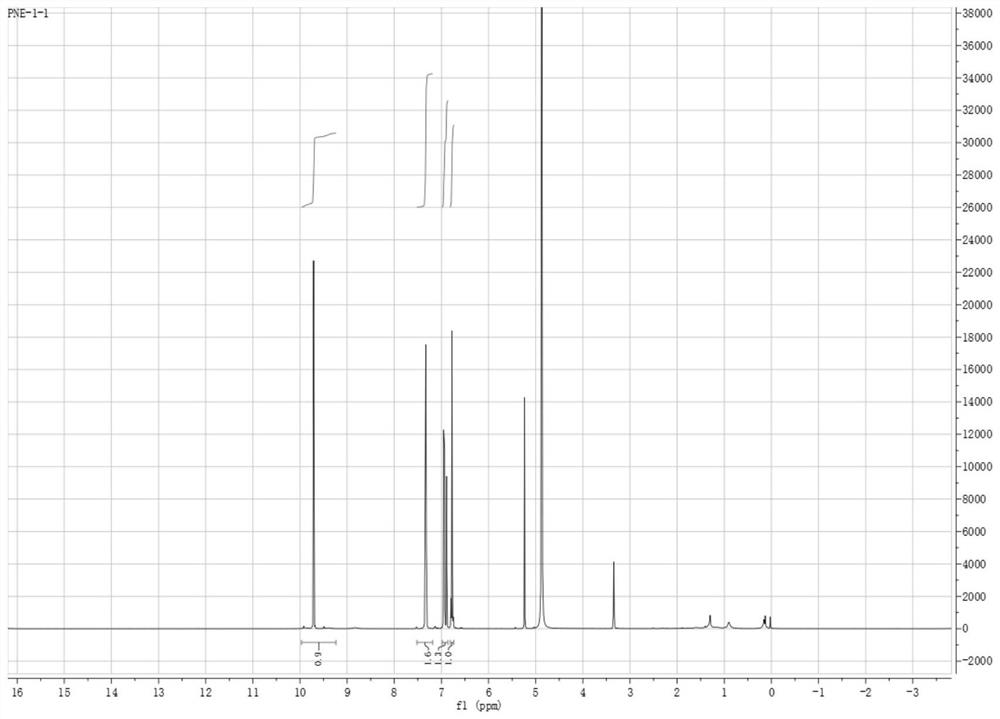
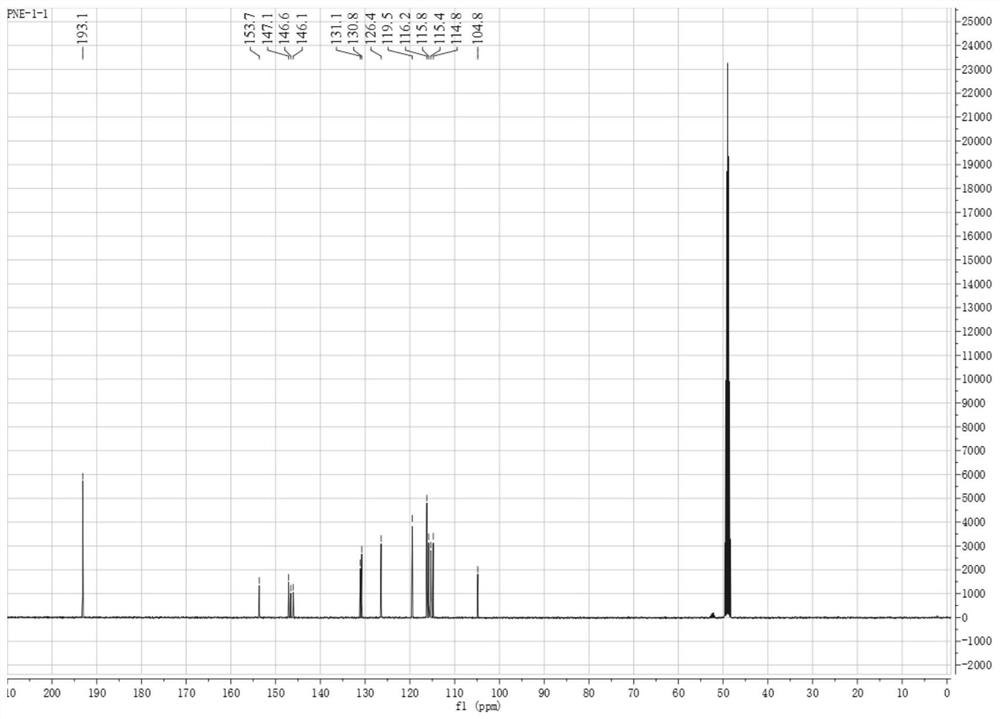
[0053] Example 1 Extraction of 2-(3',4'-dihydroxyphenyl)-1,3-piperonin-5-aldehyde
[0054] In this embodiment, the extraction of 2-(3',4'-dihydroxyphenyl)-1,3-piperonin-5-aldehyde includes the following steps:
[0055] (1) Soak 10kg lotus seed heart in 80% (v / v) ethanol for 12h, then heat and reflux for extraction 3 times, the extraction temperature is (specific value) ℃, each time for 2h, remove the insolubles by vacuum filtration while hot , and the filtrate was dried under reduced pressure to obtain the first extract. Wherein, the mass ratio of lotus seed heart and ethanol is 1:12.
[0056] (2) The first extract was sequentially extracted three times with equal volumes of petroleum ether, ethyl acetate and n-butanol, and the ethyl acetate extract was taken, dried under reduced pressure in vacuum to obtain 4.5 g of the ethyl acetate extract;
[0057] (3) The chemical components of the ethyl acetate extract were separated by repeated silica gel column chromatography, open O...
Embodiment 2
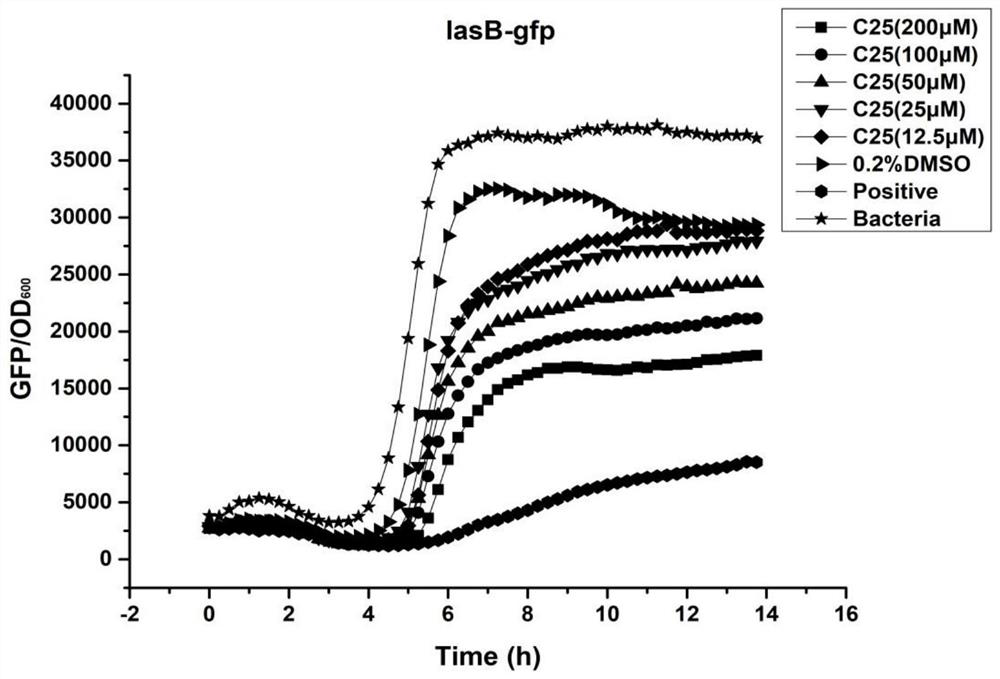
[0059] Example 2 Effect of 2-(3',4'-dihydroxyphenyl)-1,3-piperon-5-aldehyde on PAO1-lasB pathway
[0060] Test compound: the compound 2-(3',4'-dihydroxyphenyl)-1,3-piperonen-5-aldehyde was prepared into a 200mM stock solution with 100% DMSO, and was mixed with isothiocyanate The compound Iberin (10 μM) was used as a positive control, and 0.2% DMSO was used as a solvent control.
[0061] Experimental method: the compound of the present invention is mixed with ABTGC medium in a 96-well microtiter plate and mixed by a doubling dilution method to finally have 100 μL of drug-containing ABTGC medium in each well. The culture of PAO1-lasB-gfp strain grown in LB medium at 37°C and 200rpm for 12-16h was diluted in ABTGC medium to 600nm (OD 600 ) with a final optical density of 0.02 (2.5×10 8 CFU / mL). Next, 100 μL of bacterial suspension was added to each well of a 96-well microtiter plate, so that the final concentration of the compound of the present invention in each well was 200,...
Embodiment 3
[0064] Example 3: Effect of 2-(3',4'-dihydroxyphenyl)-1,3-piperonin-5-aldehyde on PAO1-pqsA pathway
[0065] Test compound: the compound 2-(3',4'-dihydroxyphenyl)-1,3-piperonen-5-aldehyde was prepared into a 200mM stock solution with 100% DMSO, and was mixed with isothiocyanate The compound Iberin was used as a positive control, and 0.2% DMSO was used as a solvent control.
[0066] Experimental method: Take the overnight cultured PAO1-pqsA-gfp green fluorescent protein reporter strain and repeat the steps of the experimental method in Example 2 to detect 2-(3',4'-dihydroxyphenyl)-1,3-piperonylcycline-5 -Effects of aldehydes on the pqsA signaling pathway of the quorum sensing system of Pseudomonas aeruginosa.
[0067] Experimental results: see Figure 5 , is the inhibitory effect of 2-(3',4'-dihydroxyphenyl)-1,3-piperonen-5-aldehyde on the expression of pqsA-gfp in Example 3 of the present invention. Figure 5 It shows that 2-(3',4'-dihydroxyphenyl)-1,3-piperonyl-5-aldehyde ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com