Lactobacillus fermentum with effect of relieving osteoporosis and application thereof
A technology of Lactobacillus fermentum and osteoporosis, applied in the field of microorganisms, can solve the problems of poor universality, patient dependence and tolerance, embolism, etc., and achieve the effect of strong universality and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1: the acquisition of Lactobacillus fermentum
[0079] Specific steps are as follows:
[0080] Take fresh feces from healthy people in Enshi, Hubei as samples, add 0.5mL sample to 5mL MRS liquid medium, and incubate at 37°C for 18-24h to obtain enriched samples; draw 0.5mL enriched The pooled sample was added to 4.5mL sterile saline to obtain 10 -1 Diluent, then draw 0.5mL 10 -1 Diluted in 4.5mL of normal saline to obtain 10 -2 Diluent, according to this operation, successively get 10 -3 , 10 -4 , 10 -5 , 10 -6 Dilution; pipette 100 μL of gradient dilution and spread on MRS solid medium, 10 -4 , 10 -5 , 10 -6 1 plate for each gradient, cultivated at 37°C for 48 hours to obtain colonies; select colonies with typical characteristics of Lactobacillus fermentum on the MRS solid medium according to the shape, size, edge, and transparency of the colonies, and use an inoculation loop to pick the colonies on the MRS solid culture Streak on the base, culture...
Embodiment 2
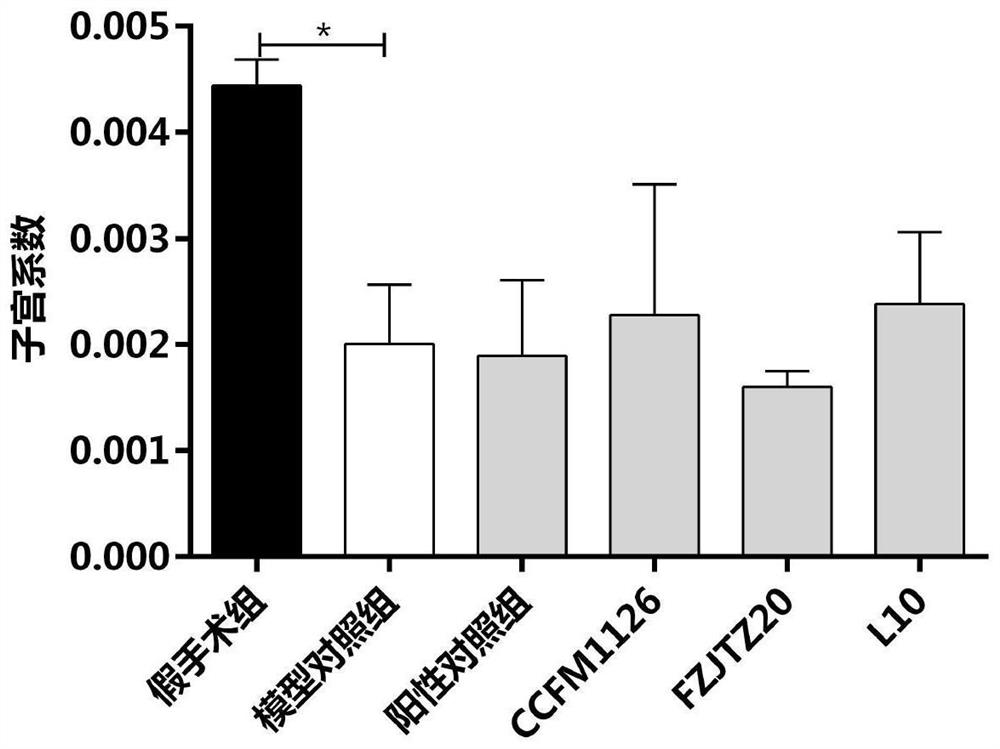
[0086] Example 2: Effect of Lactobacillus fermentum on Uterine Coefficient of Ovariectomized Rats
[0087] Specific steps are as follows:
[0088] Take 30 SPF grade female SD rats with a body weight of 250±20g, and divide them into 6 groups randomly, 5 rats in each group, and the 6 groups are: sham operation group, model control group, and positive control of intragastric administration of sodium alendronate solution group, CCFM1126 group fed with Lactobacillus fermentum CCFM1126 suspension, FZJTZ20 group fed with Lactobacillus fermentum FZJTZ20 suspension and L10 group fed with L10 suspension.
[0089] The experiment lasted for 9 weeks: the first week was the adaptation period for the rats. During the adaptation period, the rats were fed in an environment with a temperature of 22±2°C, a humidity of 40-70%, and a 12-hour cycle of day and night, free to eat and drink, and the feed used was purchased from Suzhou Shuangshi Rat breeding compound feed from Experimental Animal Feed...
Embodiment 3
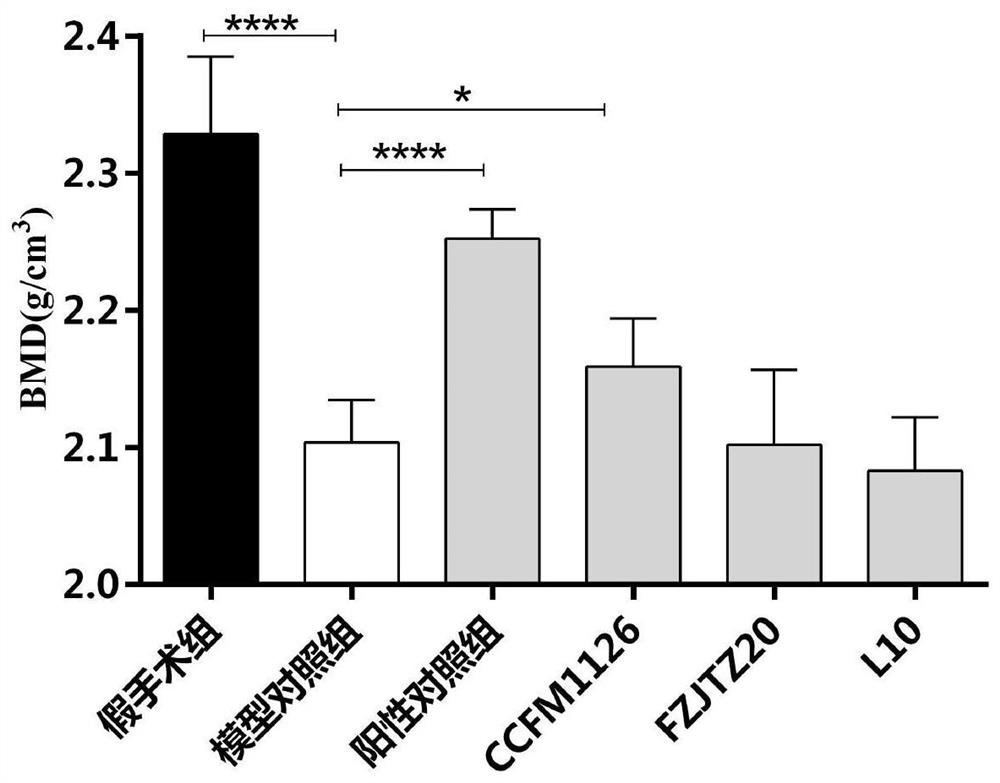
[0094] Example 3: Effects of Lactobacillus fermentum on bone mineral density and cortical volume in ovariectomized rats
[0095] Specific steps are as follows:
[0096] Take 30 SPF grade female SD rats with a body weight of 250±20g, and divide them into 6 groups randomly, 5 rats in each group, and the 6 groups are respectively: sham operation group, model control group, positive control of intragastric administration of sodium alendronate solution group, CCFM1126 group fed with Lactobacillus fermentum CCFM1126 suspension, FZJTZ20 group fed with Lactobacillus fermentum FZJTZ20 suspension and L10 group fed with L10 suspension.
[0097] The experiment lasted for 9 weeks: the first week was the adaptation period for the rats. During the adaptation period, the rats were fed in an environment with a temperature of 22±2°C, a humidity of 40-70%, and a 12-hour cycle of day and night, free to eat and drink. The feed used was purchased from Suzhou Shuangshi Rat breeding compound feed fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com