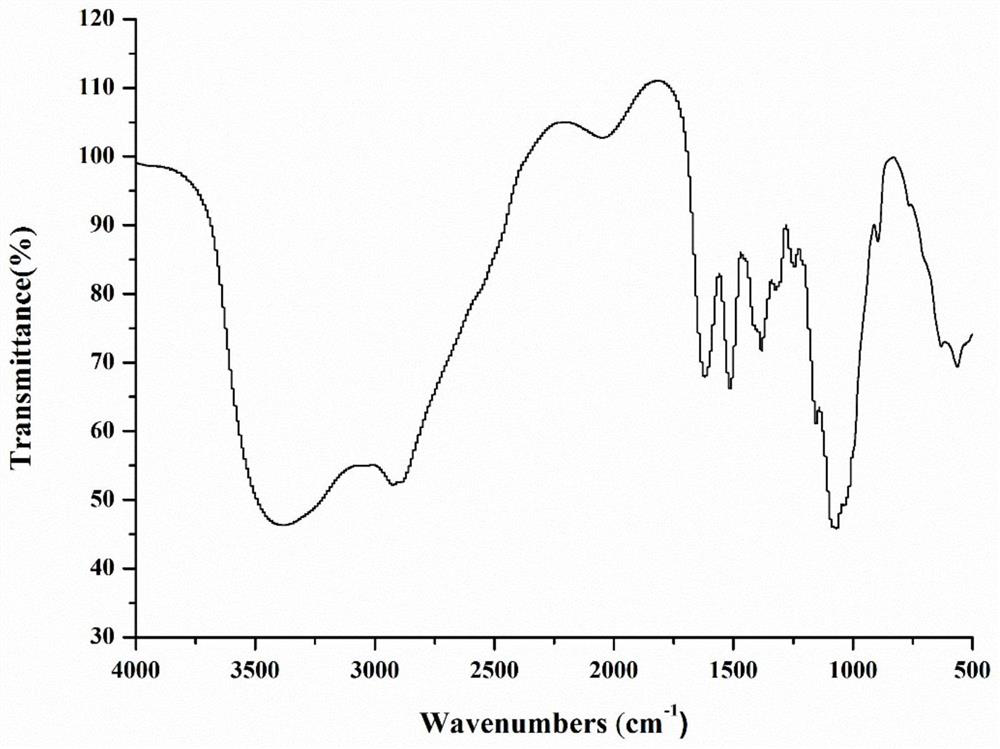
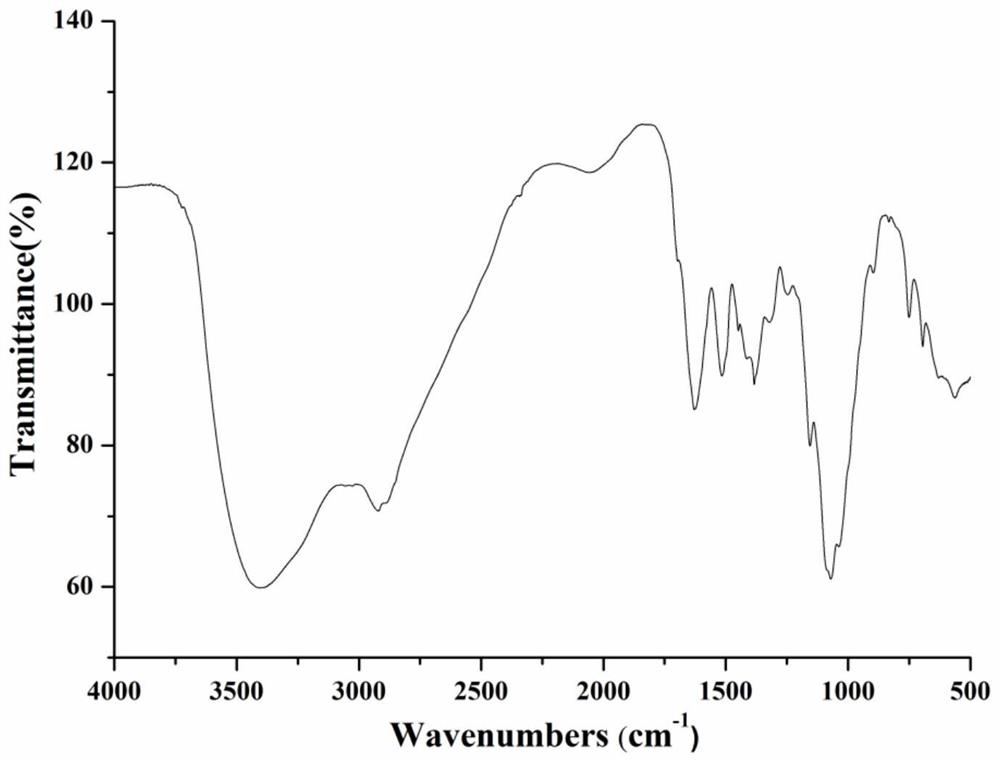
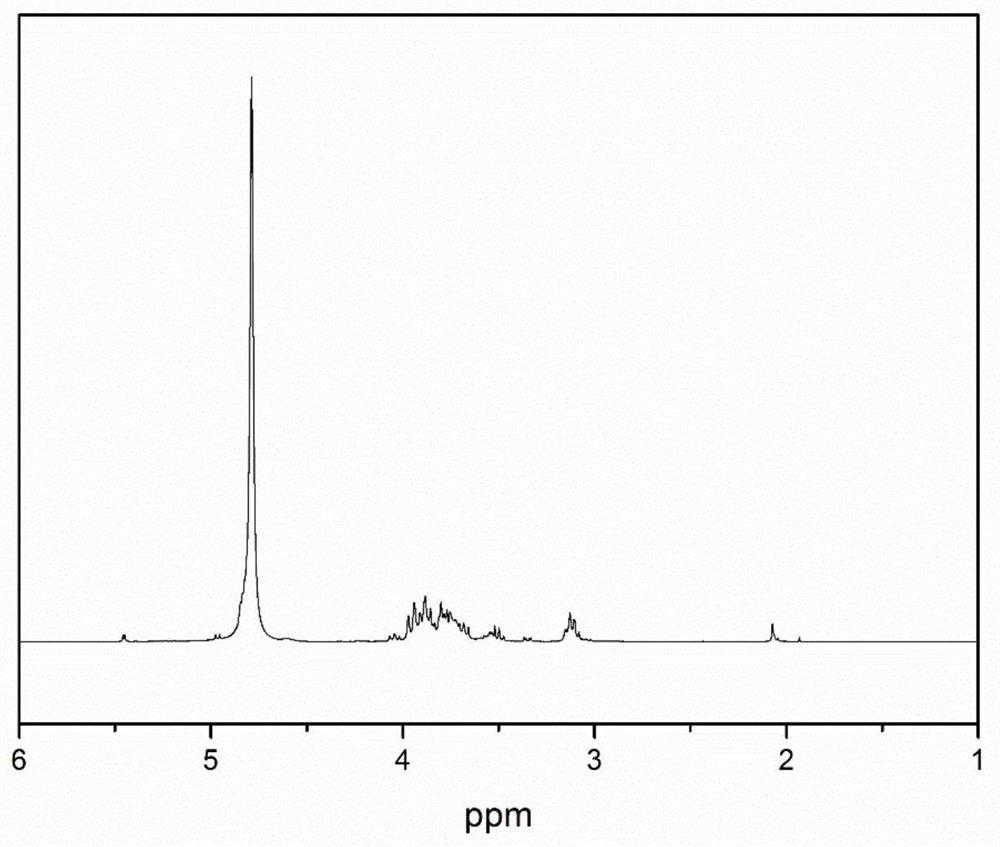
Chitosan oligosaccharide-M-cinnamyl alcohol derivative, and preparation method and application thereof
A technology of chitosan oligosaccharide and cinnamyl alcohol, applied in the field of food additives, can solve the problems of low antibacterial activity and the like, and achieve the effects of enhanced antibacterial activity, strong inhibitory effect and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation method of chitosan oligosaccharide-M-cinnamyl alcohol derivative of the present invention comprises the following steps:
[0041] (1) Preparation of Cinnamyl Bromide
[0042] Add 1 mol of cinnamyl alcohol to 30 mL of anhydrous ether, add dropwise 0.25 mL of pyridine, and stir at room temperature until clear and transparent to obtain a mixture of cinnamyl alcohol; under stirring in an ice bath, add 0.4 mol of phosphorus tribromide and 20 mL of diethyl ether to tribromide Phosphorus mixed solution, added dropwise to the cinnamyl alcohol mixed solution within 15 minutes; after the dropwise addition, the temperature was raised to 50°C, and after heating and stirring for 2 hours, the upper organic layer was separated into water, sodium bicarbonate solution and saturated sodium chloride solution using a separatory funnel. After washing, dry over anhydrous sodium sulfate, overnight, rotary evaporation to obtain a yellow syrupy product, that is, cinnamyl bromide...
Embodiment 2
[0054] The preparation method of chitosan oligosaccharide-M-cinnamyl alcohol derivative of the present invention comprises the following steps:
[0055] (1) Preparation of Cinnamyl Bromide
[0056] Add 1 mol of cinnamyl alcohol to 30 mL of anhydrous ether, add dropwise 0.15 to 0.25 mL of pyridine, and stir at room temperature until clear and transparent to obtain a cinnamon alcohol mixture; Phosphorus bromide mixed solution, added dropwise to the cinnamyl alcohol mixed solution within 15 minutes; after the dropwise addition, the temperature was raised to 40-50°C, and after heating and stirring for 2 hours, the upper organic layer was separated with water, sodium bicarbonate solution, and saturated After washing with sodium chloride solution, drying with anhydrous sodium sulfate, overnight, rotary evaporation to obtain a yellow syrupy product, that is, cinnamyl bromide.
[0057] (2) Preparation of oligochitosan-M-cinnamyl alcohol derivatives
[0058] Select chitosan oligosacc...
Embodiment 3
[0063] The preparation method of chitosan oligosaccharide-M-cinnamyl alcohol derivative of the present invention comprises the following steps:
[0064] (1) Preparation of Cinnamyl Bromide
[0065] Add 1 mol of cinnamyl alcohol to 30 mL of anhydrous ether, add dropwise 0.15 to 0.25 mL of pyridine, and stir at room temperature until clear and transparent to obtain a cinnamon alcohol mixture; Phosphorus bromide mixed solution, added dropwise to the cinnamyl alcohol mixed solution within 15 minutes; after the dropwise addition, the temperature was raised to 40-50°C, and after heating and stirring for 2 hours, the upper organic layer was separated with water, sodium bicarbonate solution, and saturated After washing with sodium chloride solution, drying with anhydrous sodium sulfate, overnight, rotary evaporation to obtain a yellow syrupy product, that is, cinnamyl bromide.
[0066] (2) Preparation of oligochitosan-M-cinnamyl alcohol derivatives
[0067] Select oligochitosacchari...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com