A kind of method of alkane selective catalytic oxidation reaction
A catalytic oxidation and selective technology, applied in catalytic reactions, chemical instruments and methods, catalysts, etc., can solve the problem of large amount of catalyst, and achieve the effect of improving catalytic efficiency, high efficiency and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
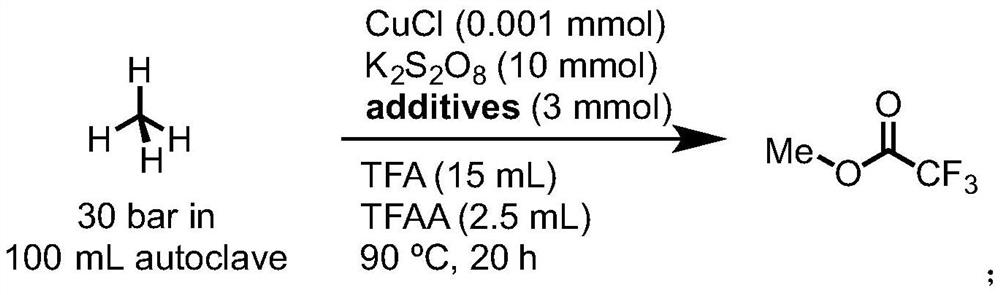
[0040] This example takes methane as an example to explore the method of preparing methyl trifluoroacetate by catalytic oxidation of methane. As a catalytic system, react in the presence of oxidant potassium persulfate to prepare methyl trifluoroacetate.
[0041]
[0042] Explore the influence of each reaction condition on the reaction in the above reaction process:
[0043] 1. Explore the reactivity of different metal salts
[0044] Using methane as raw material, cuprous chloride and different kinds of metal salt additives as catalyst system, the reaction is carried out. The specific reaction formula is as follows:
[0045]
[0046] The reaction pressure of methane is 30bar, and the catalyst is CuCl (0.5M in conc.HCl) which is newly prepared, and the amount added in the reaction is 10ul, 0.001mmol, K 2 S 2 o 8 The amount used is 10 mmol, not particularly limited. The amount of metal salt used is 3 mmol, the amount of TFA added is 15 mL, the amount of TFAA added is 2...
Embodiment 2
[0088] The optimal test conditions explored in Example 1 are extended to the oxidation reactions of other alkanes, as follows:
[0089]
[0090] The above method is also applicable to the catalytic oxidation reaction of other alkanes, and can also prepare selective oxidation products.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com