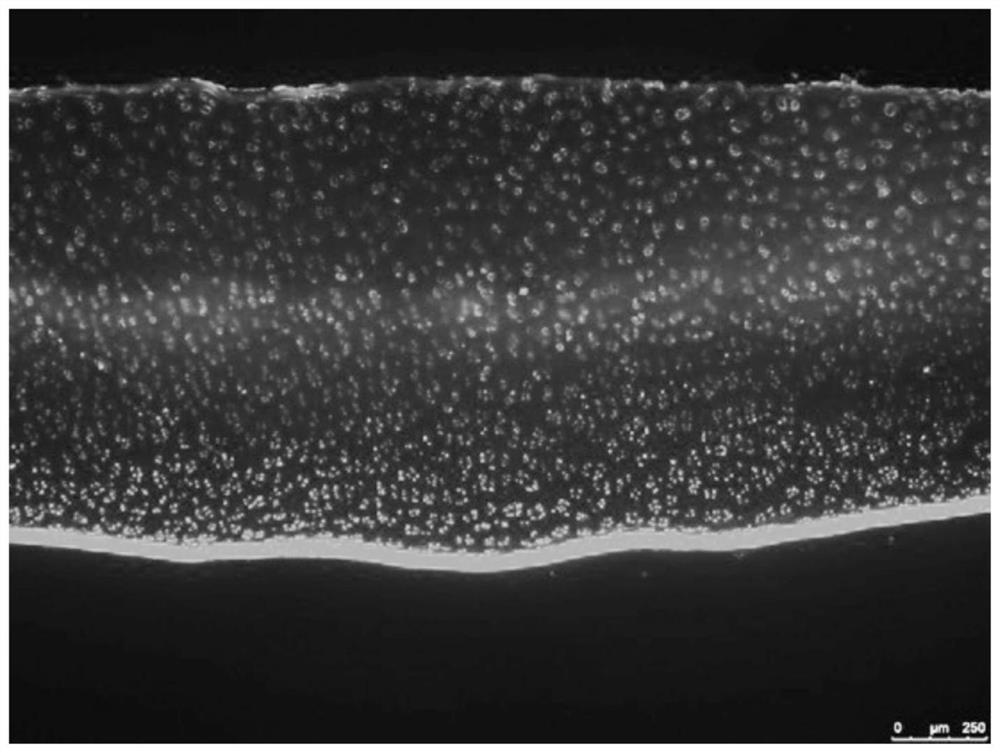
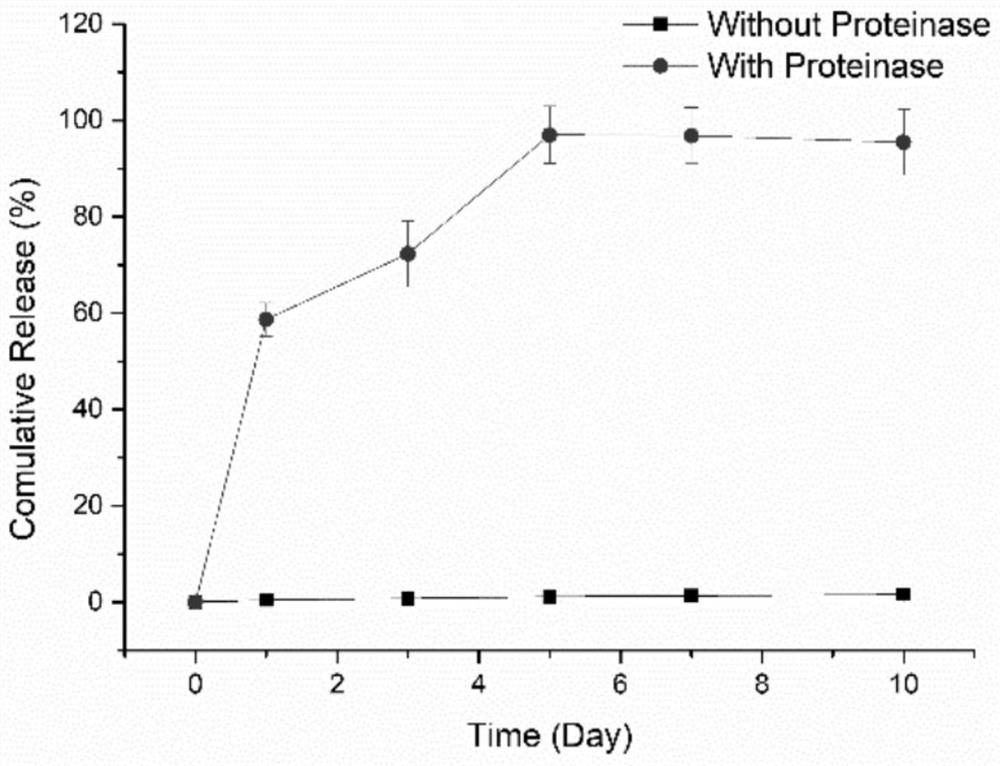
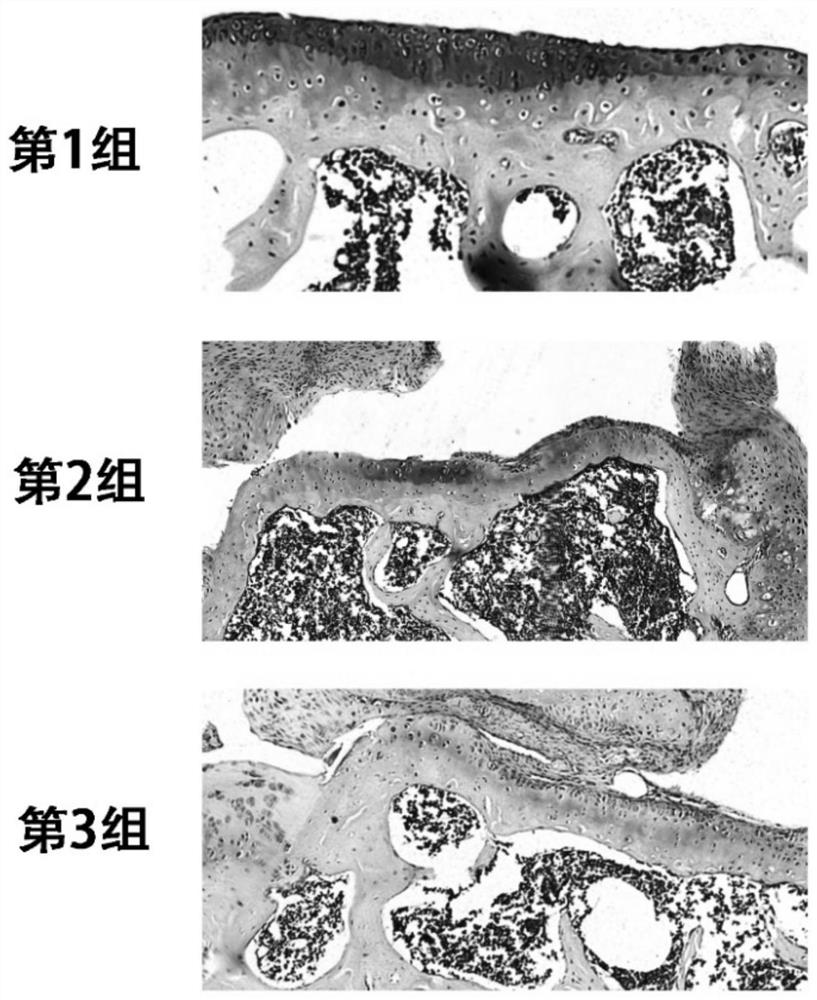
Kartogenin cartilage delivery material based on epsilon-polylysine as well as preparation method and kit thereof
A technology of polylysine and polylysine hydrochloride, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, bone diseases, etc., can solve the problem that it is difficult to really improve KGN intervention in arthritis biological Utilization etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] This embodiment provides a Kartogenin cartilage delivery material based on ε-polylysine, which has a structure such as formula (I):
[0051]
[0052] Among them, R is:
[0053]
[0054] R 1 for:
[0055]
[0056] Among them, p and q are the collection of positive integers and 0, and 0≤p≤50, 0≤q≤20;
[0057] R 2 for: -H or
[0058]
[0059]
[0060] In a preferred embodiment, in formula (I), n is a positive integer, and 5≤n≤200.
[0061] In a preferred embodiment, in formula (I), a, b, and c are a set of positive integers and 0, and a≥0, and at least one of b and c is not 0.
[0062] In a preferred embodiment, in formula (I), a+b+c=n.
Embodiment 2
[0064] This embodiment provides the preparation method of the ε-polylysine-based Kartogenin cartilage delivery material having the structure of formula (I) when c=0 in the above-mentioned embodiment 1:
[0065] Accurately weigh 0.317g (1mmol) KGN and 0.26g (1.2mmol) 4-(4,6-dimethoxytriazin-2-yl)-4-methylmorpholine hydrochloride (DMTMM), fully dissolve In 15mL of DMF, stirred at 25°C for 2 hours; then, under the conditions of 5Pa and 60°C, the organic solvent DMF was evaporated, and the obtained solid powder was dissolved in 2mL of dichloromethane, and separated by column chromatography. The separation conditions were: : 200-300-mesh column chromatography silica gel, the eluent is a mixed solvent of dichloromethane / petroleum ether with a volume ratio of 1:2, and the yellow powder active intermediate with the structure of formula (II) is obtained.
[0066]
[0067] Accurately weigh 1g (the molar weight of the amino group is about 6.1mmol) of ε-polylysine hydrochloride (the de...
Embodiment 3
[0071] This example provides the preparation method of the epsilon-polylysine-based Kartogenin cartilage delivery material having the structure of formula (I) when a=0.6, b=0.2, and c=0.2 in the above-mentioned embodiment 1:
[0072] Kartogenin (0.32g, 0.1mmol), PEG polymer represented by formula (III) (p=5, q=1, 0.36g, 0.12mmol), dicyclohexylcarbodiimide (0.25g, 0.12mmol) and 4-dimethylaminopyridine (0.15g, 0.12mmol) were dissolved in anhydrous dichloromethane, stirred at 25°C for 25 hours; Compound 0.5g, yield 80%;
[0073]
[0074] Accurately weigh 1 g of polylysine hydrochloride (the molar weight of the amino group is about 6.1 mmol), dissolve it in 50 mL of water / N'N-dimethylformamide with a volume ratio of 1:1, and use 2M NaOH The pH of the solution was adjusted to between 8-9; then 0.56g (1.22mmol) of compound (II) and 1.4g (2.44mmol) of compound (IV) were added, and stirred at room temperature for 30 hours; then 50mL was added to the reaction solution to Ionized w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com