Synthesis method of metal salt catalyzed alpha-diketone compound
A synthesis method and metal salt technology, applied in the preparation of organic compounds, separation/purification of carbonyl compounds, preparation of carbonyl compounds, etc., can solve the problems of high reaction temperature, complicated operation, deep oxidation, etc., and achieve high yield, The synthesis method is simple and the conditions are mild
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of 6-methyl-5-heptene-3,4-dione
[0026]
[0027] In a thoroughly dry Schlenk reaction tube, add 0.0207g (0.1mmol, 2mol%) silver perchlorate (AgClO 4 ), 0.4206g (5mmol) 2-methyl-3-butyn-2-ol, 3.2535g (25mmol) propionic anhydride. Then the system was placed at 25°C under magnetic stirring for 12 hours. After the reaction was completed, the acid was removed with saturated sodium bicarbonate, and the crude product was separated by column chromatography. Pure n-hexane to n-hexane:petroleum ether=1 :1 is the eluent purification to obtain the target product 6-methyl-5-heptene-3,4-dione with a yield of 90%.
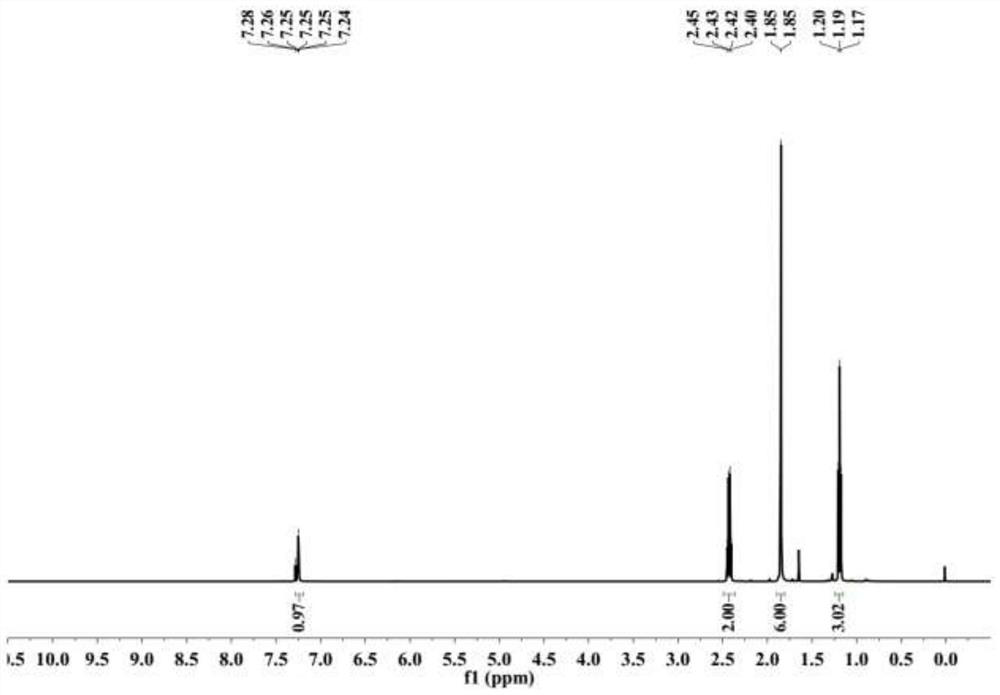
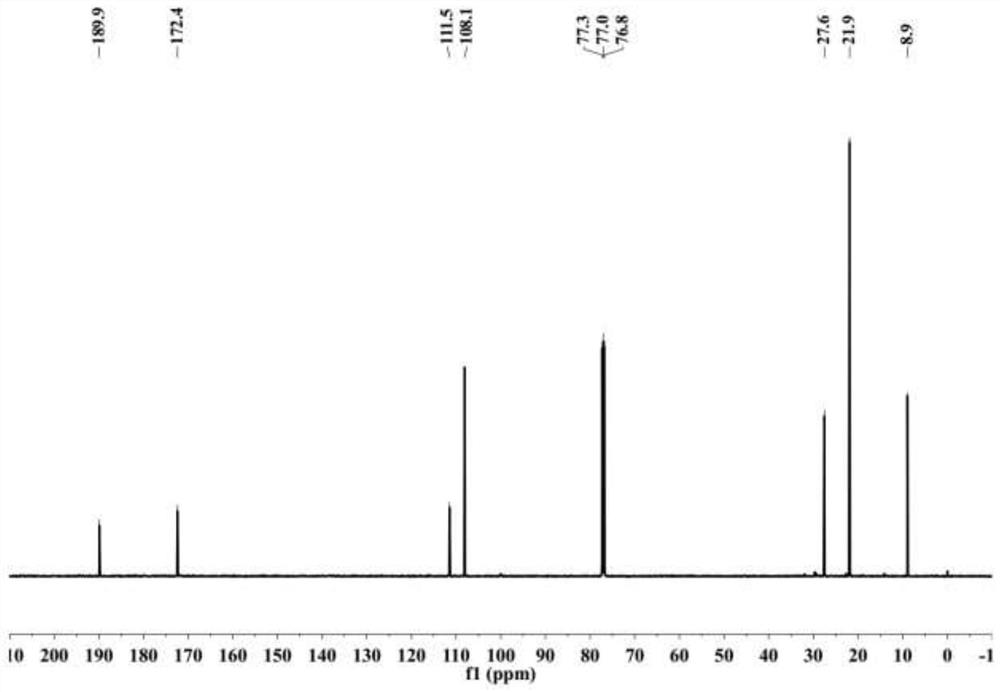
[0028] figure 1 For 6-methyl-5-heptene-3,4-dione in this embodiment 1 H NMR spectrum, figure 2 For 6-methyl-5-heptene-3,4-dione in this embodiment 13 C NMR spectrum.
[0029] from figure 1 It can be seen in: 1 H NMR (500MHz, Chloroform-d) δ7.25(m, 1H), 2.43(q, J=7.6Hz, 2H), 1.85(d, J=2.1Hz, 6H), 1.19(t, J=7.6Hz ,3H). This is consistent with the com...
Embodiment 2
[0033] Synthesis of 6-methyl-5-heptene-3,4-dione
[0034]
[0035] In a thoroughly dry Schlenk reaction tube, add 0.0207g (0.1mmol, 2mol%) silver perchlorate (AgClO 4 ), 0.4206g (5mmol) 2-methyl-3-butyn-2-ol, 3.2535g (25mmol) propionic anhydride. Then the system was placed at 25°C under magnetic stirring for 6 hours. After the reaction was completed, the acid was removed with saturated sodium bicarbonate, and the crude product was separated by column chromatography. Pure n-hexane to n-hexane:petroleum ether=1 :1 is the eluent purification to obtain the target product 6-methyl-5-heptene-3,4-dione with a yield of 88%.
Embodiment 3
[0037] Synthesis of 6-methyl-5-heptene-3,4-dione
[0038]
[0039] In a thoroughly dry Schlenk reaction tube, add 0.0104g (0.05mmol, 1mol%) silver perchlorate (AgClO 4 ), 0.4206g (5mmol) 2-methyl-3-butyn-2-ol, 0.6507g (5mmol) propionic anhydride. Then the system was placed at 25°C under magnetic stirring for 12 hours. After the reaction was completed, the acid was removed with saturated sodium bicarbonate, and the crude product was separated by column chromatography. Pure n-hexane to n-hexane:petroleum ether=1 :1 is the eluent purification to obtain the target product 6-methyl-5-heptene-3,4-dione with a yield of 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com