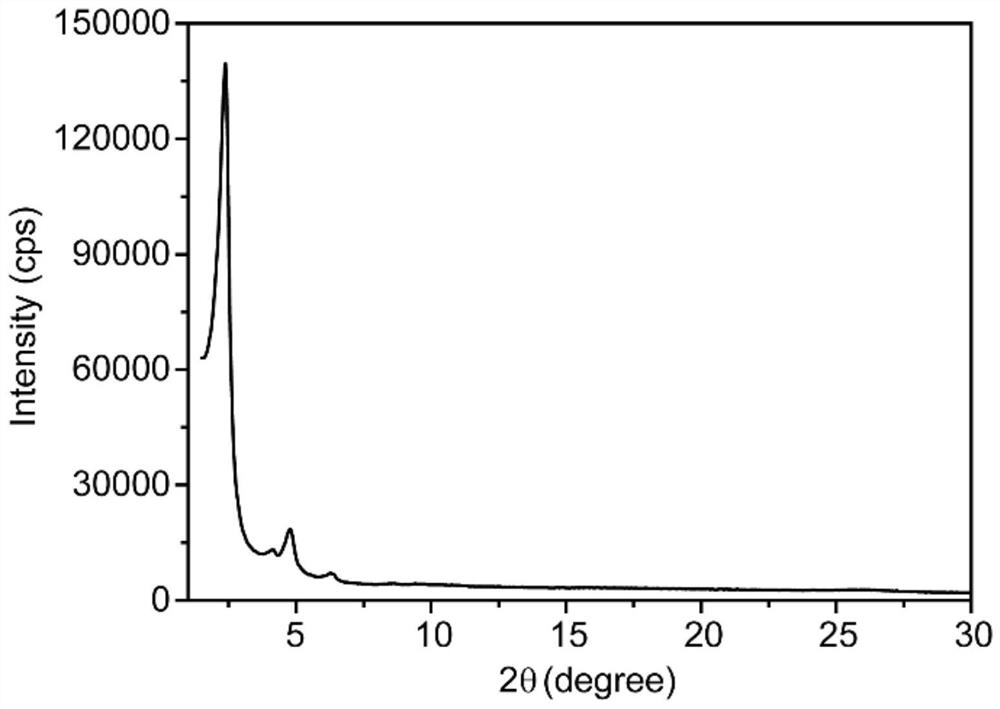
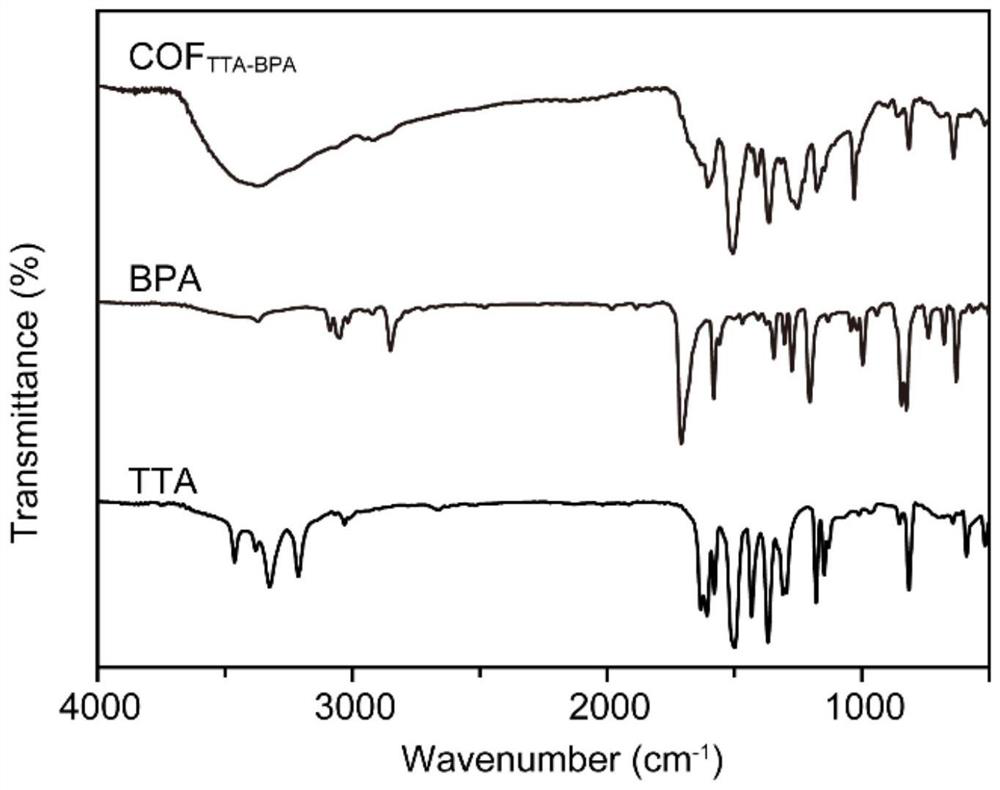
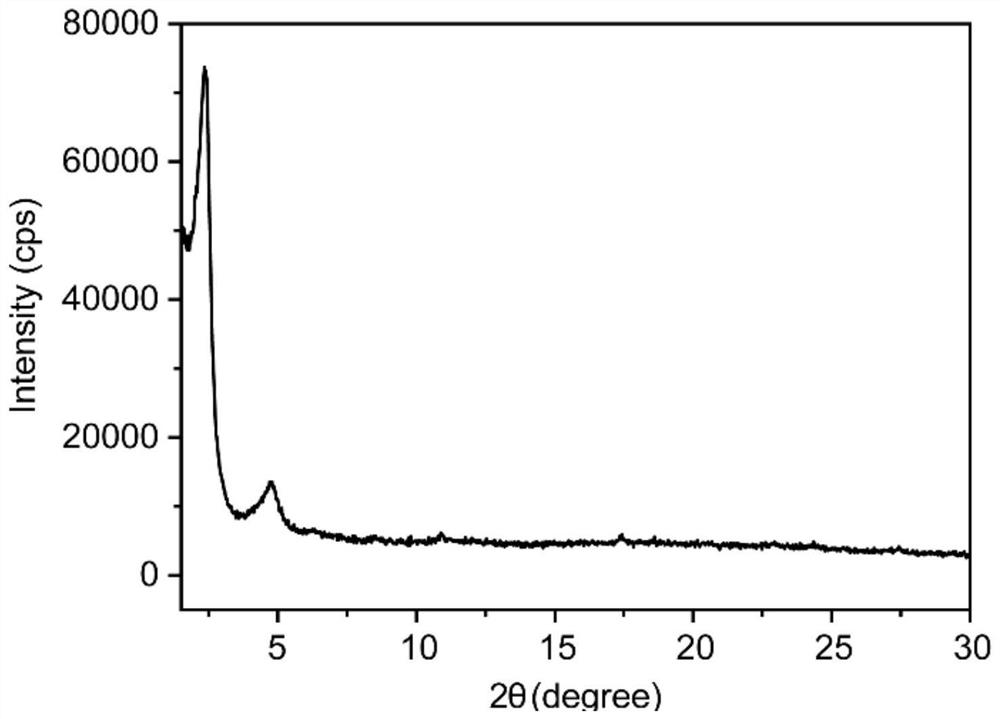
Method for rapidly preparing two-dimensional covalent organic framework material at low temperature and two-dimensional covalent organic framework material
A covalent organic framework, room temperature technology, applied in the field of two-dimensional covalent organic framework materials, low-temperature rapid preparation of two-dimensional covalent organic framework materials, can solve complex operation process, high reaction temperature, long reaction time and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This example provides a method for rapidly preparing a two-dimensional covalent organic framework material at low temperature, as follows.
[0044] Step 1: In the reaction vessel, sequentially add 1 mL of dioxane and mesitylene with a volume ratio of 4:1 mixed solvent, 0.08 mmol 4,4',4"-(1,3,5-triazine-2 ,4,6-triyl)trianiline (4,4',4"-(1,3,5-triazine-2,4,6-triyl)trianiline, TTA) and 0.12 mmol 3,3'-bipyridine -6,6'-dicarbaldehyde ([3,3'-bipyridine]-6,6'-dicarbaldehyde, BPA), ultrasonically dispersed to obtain a mixed solution 1. Wherein, the reaction container may be a glass vessel with a lid. Specifically, the ultrasonic dispersion can be 60W for 10 seconds.
[0045] Step 2: Add 0.08 mmol of Lewis acid and 0.4 mL of acetic acid solution with a concentration of 6 mol / L into the mixed solution 1, and disperse uniformly by ultrasonic to obtain the mixed solution 2. Specifically, the ultrasonic dispersion can be 60W for 5 seconds.
[0046] Step 3: Seal the reaction vess...
Embodiment 2
[0055] This example provides a method for rapidly preparing a two-dimensional covalent organic framework material at low temperature, as follows.
[0056] Step 1: In the reaction vessel, sequentially add 1 mL of dioxane and mesitylene with a volume ratio of 4:1 mixed solvent, 0.08 mmol of 1,3,5-tris(4-aminophenyl)benzene (1,3, 5-tris(4-aminophenyl)benzene, TAPB) and 0.12mmol 3,3'-bipyridine-6,6'-dicarbaldehyde (BPA) were dispersed uniformly by ultrasonic to obtain a mixed solution 1.
[0057] Step 2: Add 0.08 mmol of Lewis acid and 0.4 mL of acetic acid solution with a concentration of 6 mol / L into the mixed solution 1, and disperse uniformly by ultrasonic to obtain the mixed solution 2.
[0058] Step 3: Seal the reaction vessel containing the mixed solution 2 at normal temperature and pressure, or seal it under vacuum after quick freezing with liquid nitrogen.
[0059] Step 4: Reacting the closed reaction vessel at a relatively low temperature (0-25° C.) for 1-30 minutes to ...
Embodiment 3
[0067] This example provides a method for rapidly preparing a two-dimensional covalent organic framework material at low temperature, as follows.
[0068] Step 1: In the reaction vessel, sequentially add 1 mL of dioxane and mesitylene with a volume ratio of 4:1 mixed solvent, 0.08 mmol 4,4',4"-(1,3,5-triazine-2, 4,6-triyl)triphenylamine (TTA) and 0.12 mmol 2,5-dicarboxyphenazine (pyrazine-2,5-dicarbaldehyde, PDA) were dispersed uniformly by ultrasonic to obtain a mixed solution 1.
[0069] Step 2: Add 0.08 mmol of Lewis acid and 0.4 mL of acetic acid solution with a concentration of 6 mol / L into the mixed solution 1, and disperse uniformly by ultrasonic to obtain the mixed solution 2.
[0070] Step 3: Seal the glass reaction container containing the mixed solution 2 at normal temperature and pressure, or seal it under vacuum after quick freezing with liquid nitrogen.
[0071] Step 4: Reacting the closed reaction vessel at a relatively low temperature (0-25° C.) for 1-30 minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com