Recombinant human TSG6-IFN alpha fusion protein, preparation method thereof and application of recombinant human TSG6-IFN alpha fusion protein as antiviral drug
A TSG6-IFN, TSG-6-technology, applied in antiviral agents, recombinant DNA technology, animal/human proteins, etc., can solve the problems of unfavorable clinical treatment, short half-life, and limited scope of clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
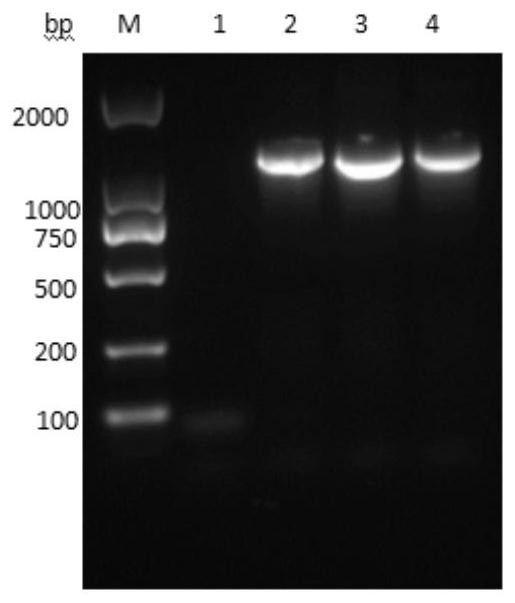
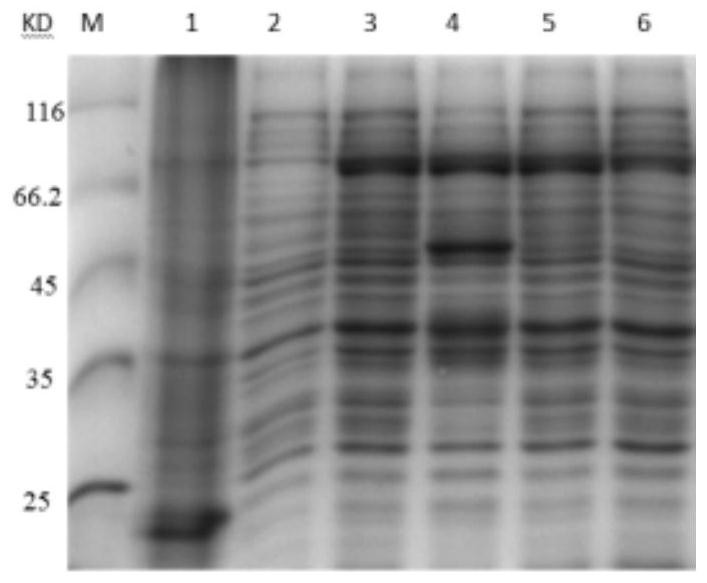
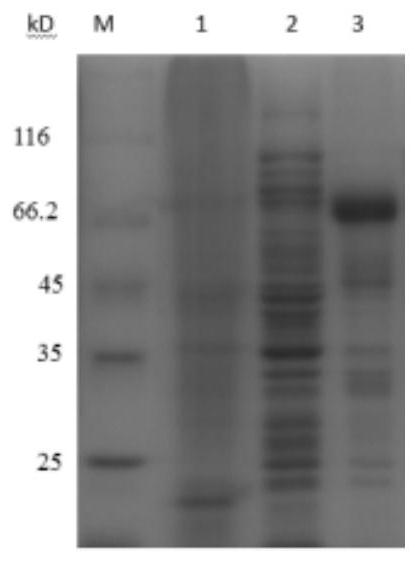
[0061] A preparation method of recombinant human TSG-6 and porcine IFN-α fusion protein (rhTSG6-PoIFNα fusion protein), including the following steps:
[0062] (1) Codon optimization of the recombinant human TSG-6 gene shown in SEQUENCE LISTING 400 by Escherichia coli codon preference to obtain the optimized recombinant human TSG shown in SEQUENCE LISTING 400 -6 gene, the amino acid sequence of recombinant human TSG-6 encoded by it is shown in SEQUENCE LISTING 400;
[0063] Codon optimization of the recombinant porcine IFN-α gene shown in SEQUENCE LISTING 400 by Escherichia coli codon preference to obtain the optimized recombinant porcine IFN-α gene shown in SEQUENCE LISTING 400 , The amino acid sequence of the encoded recombinant porcine IFN-α is shown in SEQUENCE LISTING 400 ;
[0064] Use a connecting peptide gene fragment shown in SEQUENCE LISTING 400 to combine the optimized recombinant porcine IFN-α gene shown in SEQUENCE LISTING 400 with the optimized recombination shown in S...
Embodiment 2
[0079] Determination of antiviral activity titer of rhTSG6-PoIFNα fusion protein standard
[0080] a. Experimental materials:
[0081] Recombinant human interferon alpha (rhIFN-α) standard: its titer is 15000IU / mL, as a known positive control, purchased from Beijing China Institute of Food and Drug Identification, interferon alpha national standard, batch number: 97 / 04 , Hereinafter referred to as standardized interferon;
[0082] RhTSG6-PoIFNα fusion protein product: prepared in Example 1, as the test product;
[0083] Cell line: bovine kidney cell (MDBK), purchased from ATCC;
[0084] Virus strain: The attack virus is Vesicular Stomatitis Virus (VSV), a gift from the Institute of Clinical Virology of Anhui Medical University.
[0085] b. Experimental method
[0086] Operate under aseptic conditions, take 1 rhTSG6-PoIFNα fusion protein standard and add 1mL water for injection to dissolve it, and then dilute it in a reagent bottle with DMEM cell nutrient solution containing 10% newborn c...
Embodiment 3
[0108] Determination of the half-life of rhTSG6-PoIFNα in rabbit plasma
[0109] 3.1. The half-life of rhTSG6-PoIFNα in rabbit plasma was determined. 40μg / kg recombinant porcine interferon-α (i.e. rPoIFN-α) was used as a positive control, rhTSG6-PoIFNα was injected intramuscularly into rabbits at a dose of 40μg / kg, and then blood samples (1ml) were collected at different times. Specifically: 1h before administration, 0.25h, 1h, 3h, 4h, 8h, 10h, 12h, 24h, 48h, 72h, 96h, 120h, 144h and 168h after administration, blood was collected from the ear vein of the rabbit. After the blood sample was collected, it was transferred into an EP tube containing EDTA and centrifuged together, and the supernatant was stored in a refrigerator at -80°C.
[0110] 3.2. Use the "cytopathic inhibition method" in the 2015 edition of "Chinese Pharmacopoeia" to determine the retention time of rhTSG6-PoIFNα in rabbit blood. The abscissa is the plasma collected at each time point in the rabbit, and the ordina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com