Anthracene derivative and preparation method and application thereof
An anthracene derivative and naphthalene anthracene technology, applied in the field of anthracene derivatives and their preparation, can solve the problems of insufficient color purity, intramolecular charge transfer, fluorescence quenching and the like, and achieve the effect of a simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
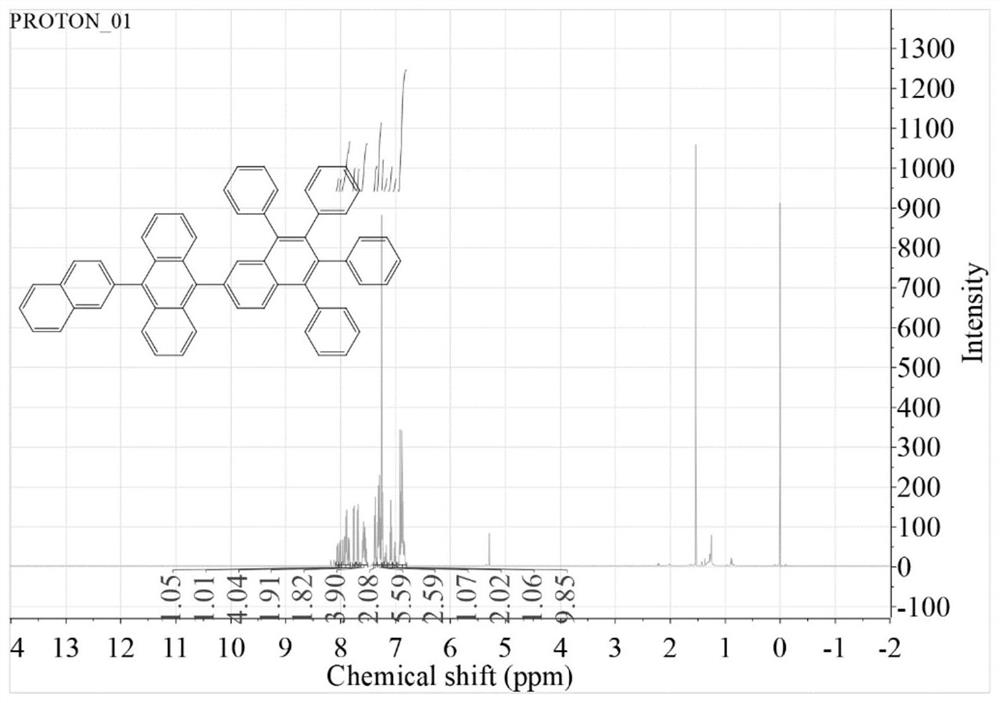
[0042] Embodiment 1 provides a kind of anthracene derivative, the preparation method of this anthracene derivative is as follows:
[0043] P-bromo-naphthalene anthracene (1.15g, 3mmol), tetraphenylnaphthaleneboronic acid (2.51g, 4.5mmol) and tetrakistriphenylphosphine palladium (0.16g, 0.21mmol) were added in a 100ml two-necked flask, and the flask was Evacuated under vacuum and replaced three times in dry nitrogen;
[0044] Add 60 mL THF and 8 mL saturated K 2 CO 3 Aqueous solution, heated to reflux and stirred at 90°C for 48 hours to obtain a mixed solution;
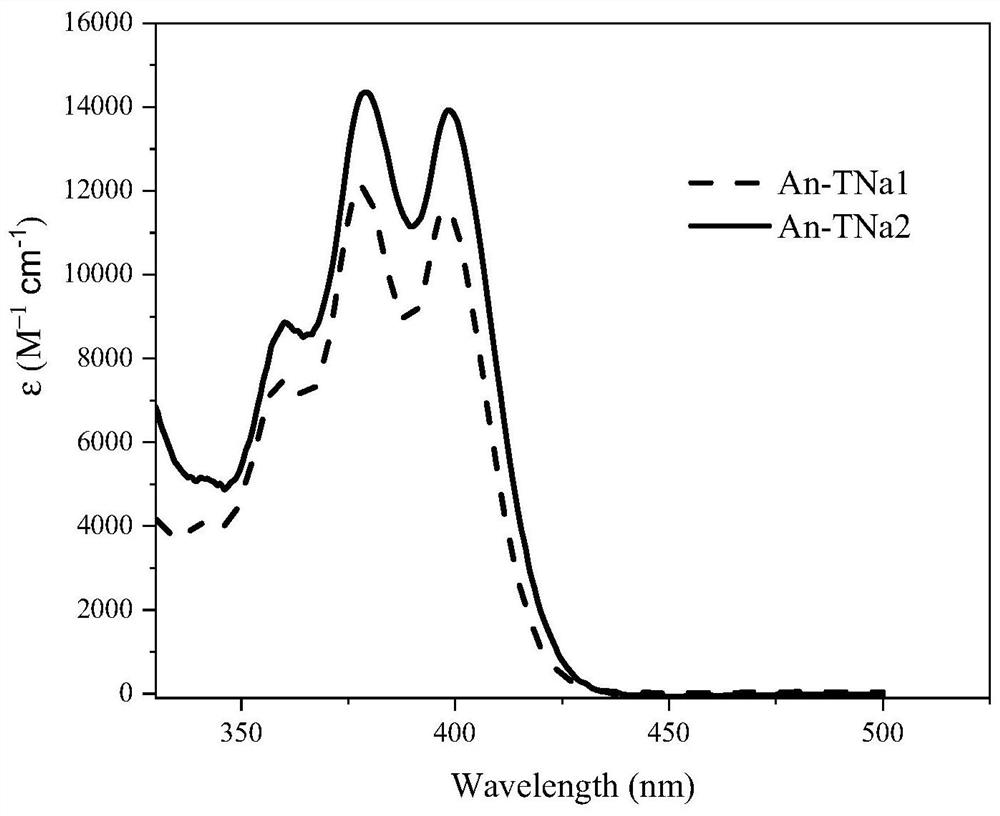
[0045] Use saturated brine and dichloromethane to extract the above mixed solution, distill under reduced pressure to obtain a black solid, which is purified by column chromatography, wherein the stationary phase is silica gel powder, and the eluent is petroleum ether / dichloromethane to obtain anthracene Derivative An-TNa1, 1.0 g in total, yield 80%.
[0046] The reaction process is as follows:
[0047]
Embodiment 2
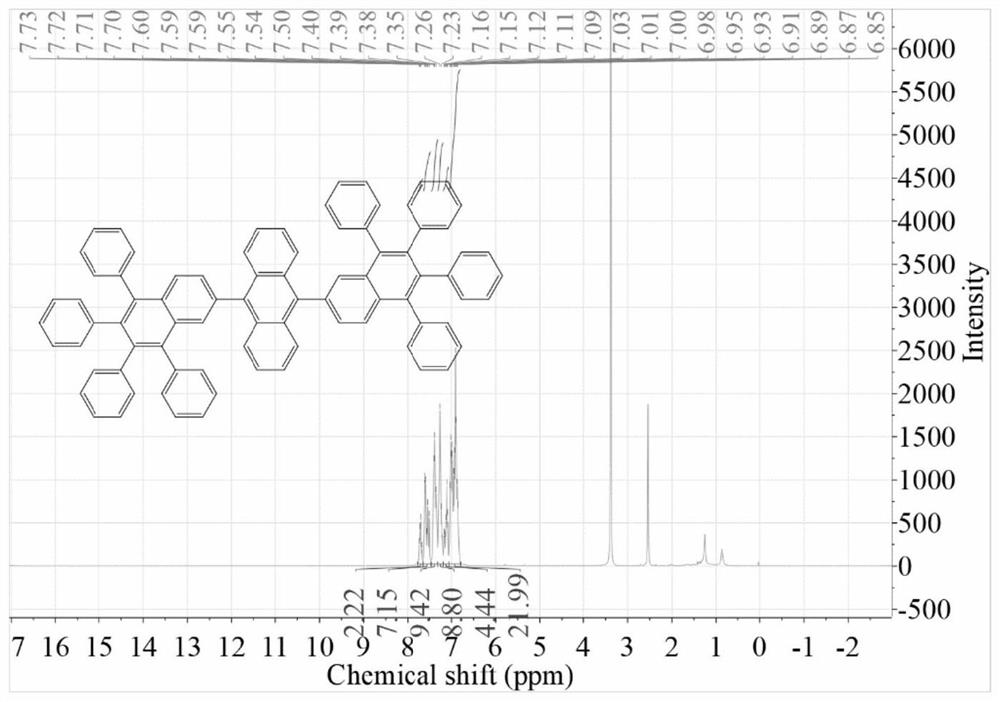
[0049] Embodiment 2 provides a kind of anthracene derivative, the preparation method of this anthracene derivative is as follows:
[0050] Two-p-bromoanthracene (1.01g, 3mmol), tetraphenylnaphthaleneboronic acid (5.02g, 9mmol) and tetrakistriphenylphosphine palladium (0.16g, 0.21mmol) were added in a 100ml two-necked flask, and the flask was placed under vacuum Evacuate and replace three times in dry nitrogen;
[0051] Add 60 mL THF and 8 mL saturated K 2 CO 3 Aqueous solution, heated to reflux and stirred at 90°C for 48 hours to obtain a mixed solution;
[0052]Use saturated brine and dichloromethane to extract the above mixed solution, and distill under reduced pressure to obtain a black solid; it is purified by column chromatography, wherein the stationary phase is silica gel powder, and the eluent is petroleum ether / dichloromethane to obtain anthracene Derivative An-TNa2, a total of 1.69g, yield 66.7%.
[0053] The reaction process is as follows:
[0054]
Embodiment 3
[0056] Example 3 provides an anthracene derivative. The difference between its preparation method and Example 1 is that the addition amount of tetraphenylnaphthalene boronic acid is 3 mmol, and the addition amount of tetrakistriphenylphosphine palladium is 0.15 mmol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com