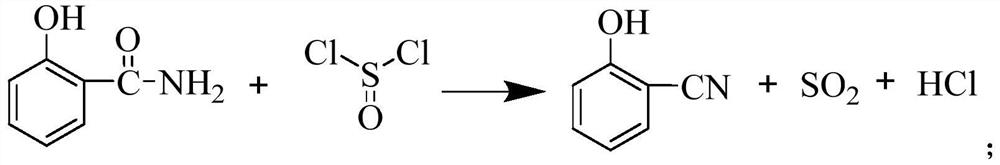
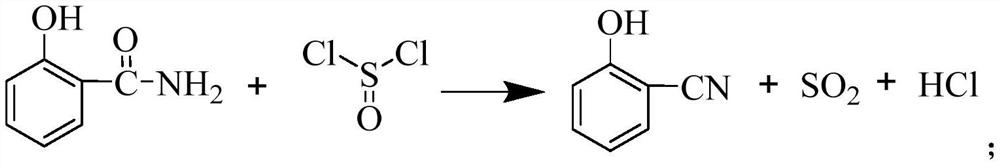
Preparation method of salicylonitrile
A technology of salicylonitrile and salicylamide, applied in the field of organic compound preparation, can solve problems such as difficult operation, influence on product yield and purity, influence on subsequent use of salicylonitrile, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The present embodiment provides a kind of preparation method of salicylonitrile, specifically comprises the following steps:
[0045] (1) Add 20g of salicylamide and 200g of xylene into a four-necked flask, stir and heat up to an internal temperature of 105°C; then slowly add thionyl chloride dropwise to the four-necked flask under stirring, and the dropping time is 5 hour, the dropwise amount of thionyl chloride was 20.67g; after the dropwise addition, continue to incubate at 105°C for 2 hours, take a sample and measure the remaining 2% of salicylamide in the reaction system as the reaction end point, and obtain salicylonitrile xylene liquid ;
[0046] (2) Add 0.21 g of thionyl chloride to the salicylonitrile xylene liquid obtained in step (1), cool down to 5° C., and salicylonitrile crystallizes out; the crystals are filtered and dried to obtain the product salicylonitrile 16.33 g, the content of salicylonitrile is 97%, the residual salicylamide is 0.08%, and the yie...
Embodiment 2
[0048] The present embodiment provides a kind of preparation method of salicylonitrile, specifically comprises the following steps:
[0049] (1) Add 20g of salicylamide and 100g of xylene into a four-necked flask, stir and heat up to an internal temperature of 125°C; then slowly add thionyl chloride dropwise to the four-necked flask under stirring, and the dropping time is 10 hour, the dropwise amount of thionyl chloride was 24.67g; after the dropwise addition, continue to incubate at 125°C for 2 hours, take a sample and measure the remaining 2% of salicylamide in the reaction system as the reaction end point, and obtain salicylonitrile xylene liquid ;
[0050] (2) Add 0.3 g of thionyl chloride to the salicylonitrile xylene liquid obtained in step (1), cool down to 8° C., and salicylonitrile crystallizes out; the crystals are filtered and dried to obtain the product salicylonitrile 17.01 g, the content of salicylonitrile is 97%, the residual salicylamide is 0.11%, and the yie...
Embodiment 3
[0052] The present embodiment provides a kind of preparation method of salicylonitrile, specifically comprises the following steps:
[0053] (1) Add 20g of salicylamide and 140g of xylene into a four-necked flask, stir and heat up to an internal temperature of 115°C; then slowly add thionyl chloride dropwise to the four-necked flask under stirring, and the dropping time is 4 hour, the dropwise amount of thionyl chloride was 20.67g; after the dropwise addition, continue to incubate at 115°C for 2 hours, take a sample and measure the remaining 2% of salicylamide in the reaction system as the reaction end point, and obtain salicylonitrile xylene liquid ;
[0054] (2) Add 0.4 g of thionyl chloride to the salicylonitrile xylene liquid obtained in step (1), cool down to 0° C., and salicylonitrile crystallizes out; the crystals are filtered and dried to obtain the product salicylonitrile 16.8 g, salicylonitrile content 97.1%, salicylamide residual 0.21%, yield 94.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com