Synthetic method of perfluoroisobutyronitrile
A technique for the synthesis of perfluoroisobutyronitrile, which is applied in the field of synthesis of perfluoroisobutyronitrile, can solve problems such as long routes, environmental pollution, and increased production costs, and achieve simple and safe processes, easy industrial production, and high reaction conversion rates and the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
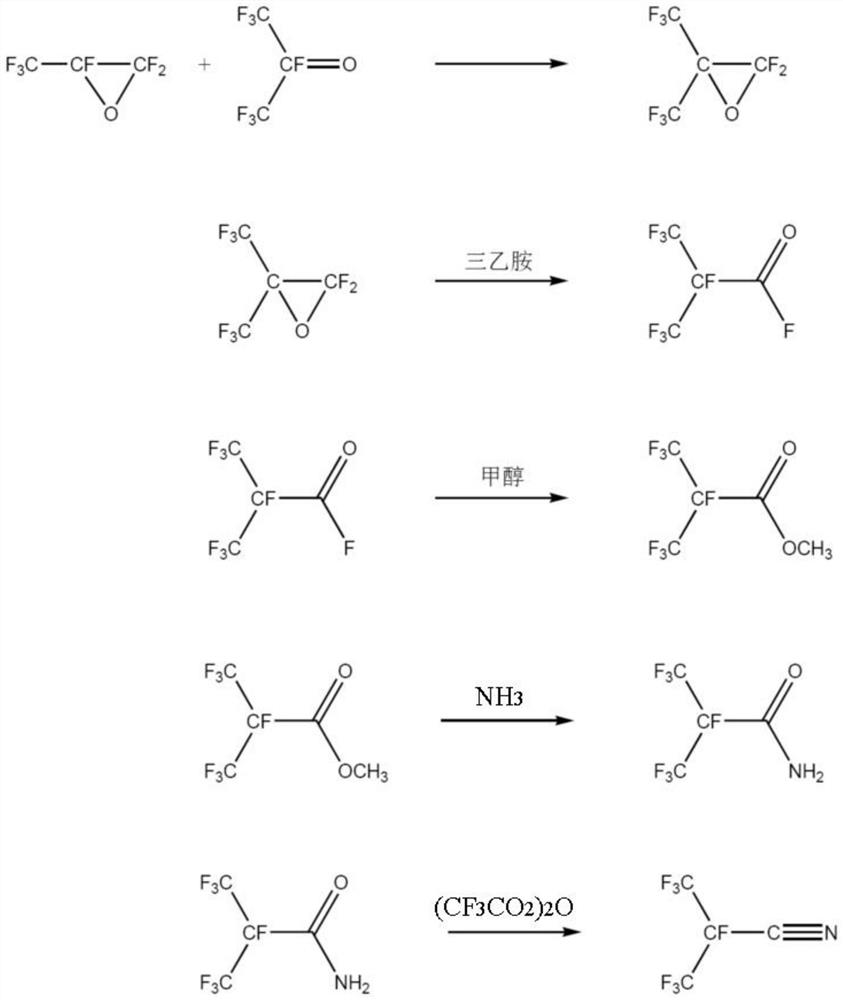
[0063] A kind of synthetic method of the perfluoroisobutyronitrile of the embodiment of the present invention, its synthetic scheme is as follows figure 1 Shown, this synthesis method comprises the following steps:
[0064] (1) Synthesis of perfluoroepoxy isobutane
[0065] First, the reaction system was heated and vacuumed to remove water, and the whole system was purged with high-purity nitrogen to remove oxygen therein; the reactor was cooled to -30°C, and hexafluoroacetone (HFA) (166g, 1mol) was metered into the reactor, Control the ventilation rate to completely liquefy the HFA; then meter in hexafluoropropylene oxide (HFPO) (166.02g, 1mol), control the ventilation rate to completely liquefy the HFPO; close the inlet valve, and raise the temperature in the kettle to 190°C for 8 hours After the reaction, the product washes the column by saturated sodium fluoride solution to obtain a mixture containing perfluoroisobutylene oxide and perfluoropropylene oxide, and distillat...
Embodiment 2
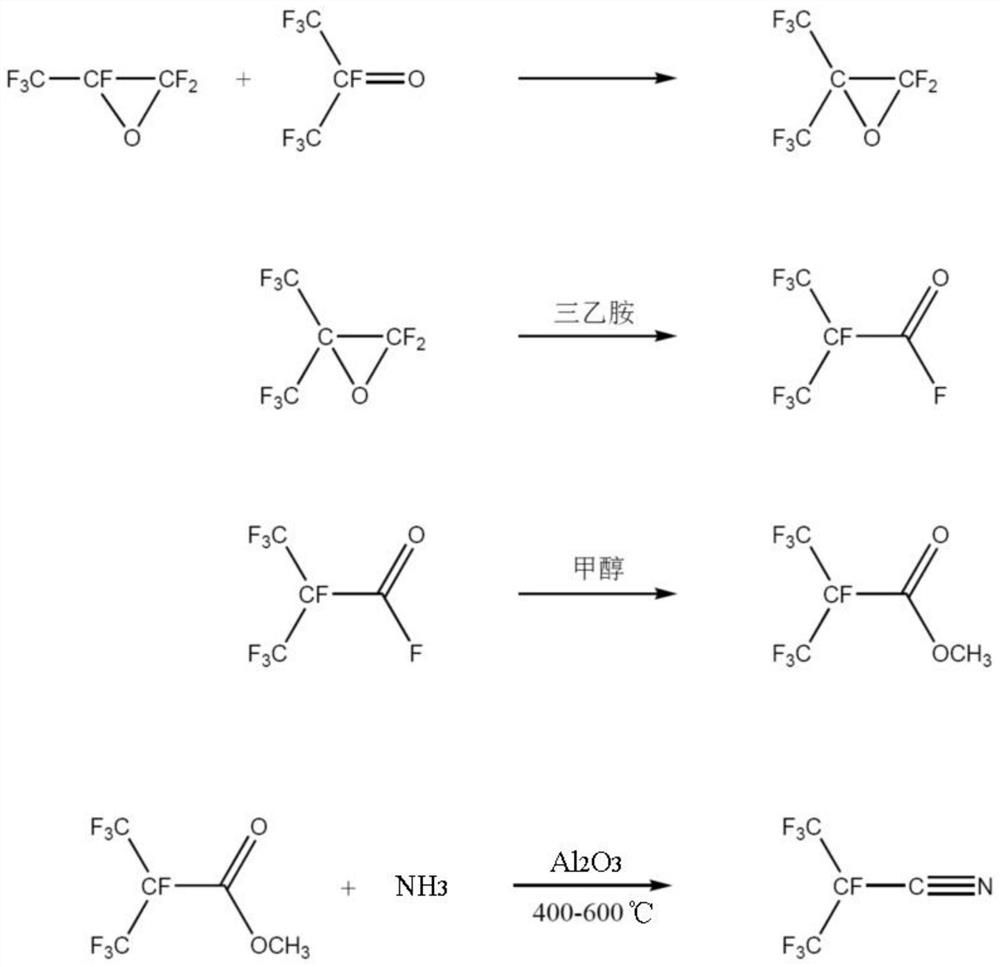
[0075] A kind of synthetic method of the perfluoroisobutyronitrile of the embodiment of the present invention, its synthetic scheme is as follows figure 2 Shown, this synthesis method comprises the following steps:
[0076] (1) Synthesis of perfluoroepoxy isobutane
[0077] First, the reaction system was heated and vacuumed to remove water, and the whole system was purged with high-purity nitrogen to remove oxygen therein; the reactor was cooled to -30°C, and hexafluoroacetone (HFA) (166g, 1mol) was metered into the reactor, Control the ventilation rate to completely liquefy the HFA; then meter in hexafluoropropylene oxide (HFPO) (166.02g, 1mol), control the ventilation rate to completely liquefy the HFPO; close the inlet valve, and raise the temperature in the kettle to 190°C for 8 hours After the reaction, the product passes through the saturated aqueous sodium fluoride washing column to obtain a mixture containing perfluoroisobutylene oxide and perfluoropropylene oxide; d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com