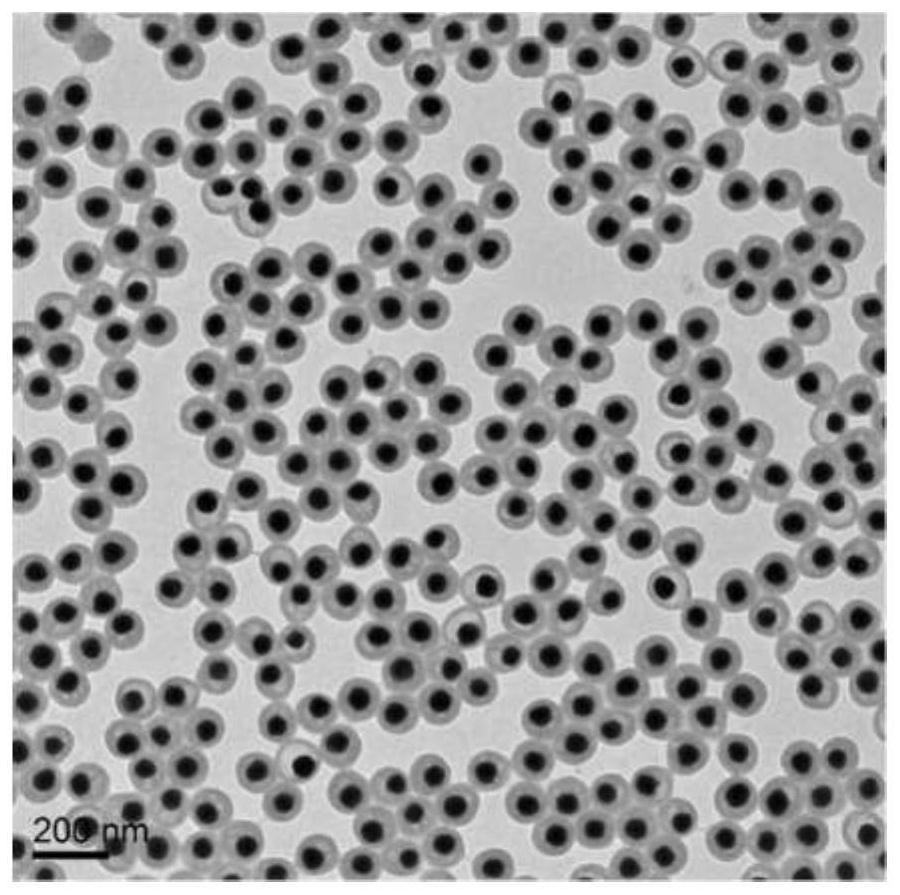
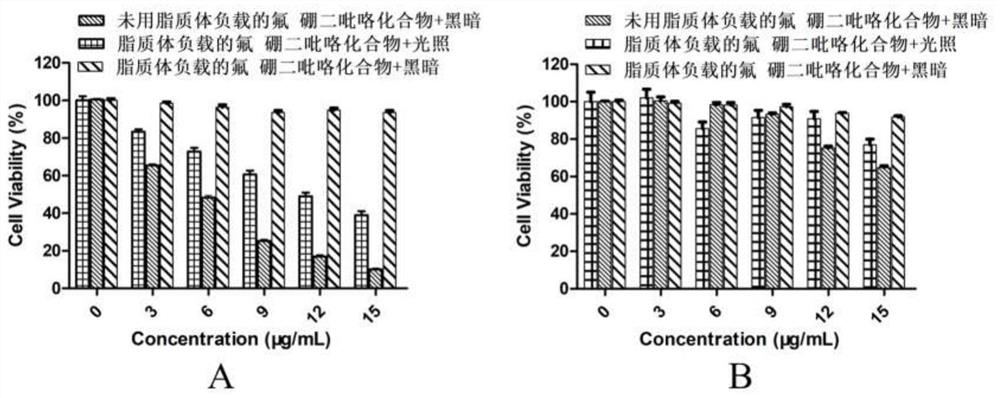
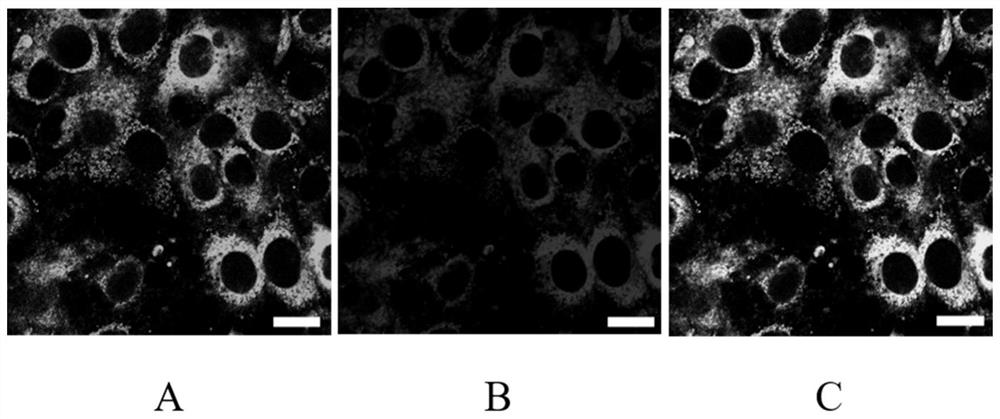
Mitochondrion-targeting BODIPY compound and preparation method and application of liposome-coated nanoparticles of BODIPY compound
A fluoroboron dipyrrole and nanoparticle technology, which is applied in the field of fluoroboron dipyrrole compounds, can solve problems such as limiting the enrichment of nanoparticles, uneven particle size distribution of nanoparticles, and inconspicuous photodynamic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthetic R 1 for-OCH 3 The specific preparation process of the modified mitochondria-targeted fluoroborate dipyrrole compound includes:
[0042] (1) Dissolve 4-methoxybenzaldehyde (409 mg, 3 mmol) in 90 mL of anhydrous tetrahydrofuran. Under stirring, 2,4-dimethylpyrrole (0.63 g, 6.6 mmol) and 0.25 mL of trifluoroacetic acid were added, and the reaction solution was reacted under nitrogen protection for 14 hours. After that, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (0.68g, 3mmol) was dissolved in 120mL of anhydrous tetrahydrofuran, and added dropwise to the above reaction solution, and the reaction was continued for 4 hours . After the above reaction solution was placed in a mixture of ice and water, 18 mL of triethylamine was added dropwise. After the reaction solution was reacted in an ice-water mixture for 30 minutes, 18 mL of boron trifluoride diethyl ether was added dropwise, and the reaction solution was continued to react at 25° C. for 14 hours. After the ...
Embodiment 2
[0056] Synthetic R 1 for -N(C 2 h 5 ) 2 The specific preparation process of the modified mitochondria-targeted fluoroborate dipyrrole compound includes:
[0057] (1) Dissolve 4-(N,N-diethyl)aminobenzaldehyde (532 mg, 3 mmol) in 90 mL of anhydrous tetrahydrofuran. Under stirring, 2,4-dimethylpyrrole (0.63 g, 6.6 mmol) and 0.25 mL of trifluoroacetic acid were added, and the reaction solution was reacted under nitrogen protection for 14 hours. After that, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (0.68g, 3mmol) was dissolved in 120mL of anhydrous tetrahydrofuran, and added dropwise to the above reaction solution, and the reaction was continued for 4 hours . After the above reaction solution was placed in a mixture of ice and water, 18 mL of triethylamine was added dropwise. After the reaction solution was reacted in an ice-water mixture for 30 minutes, 18 mL of boron trifluoride diethyl ether was added dropwise, and the reaction solution was continued to react at 25° C. for...
Embodiment 3
[0071] Synthetic R 1 for The specific preparation process of the modified mitochondria-targeted fluoroborate dipyrrole compound includes:
[0072] (1) Dissolve 4-(4-morpholine)benzaldehyde (574 mg, 3 mmol) in 90 mL of anhydrous tetrahydrofuran. Under stirring, 2,4-dimethylpyrrole (0.63 g, 6.6 mmol) and 0.25 mL of trifluoroacetic acid were added, and the reaction solution was reacted under nitrogen protection for 14 hours. After that, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (0.68g, 3mmol) was dissolved in 120mL of anhydrous tetrahydrofuran, and added dropwise to the above reaction solution, and the reaction was continued for 4 hours . After the above reaction solution was placed in a mixture of ice and water, 18 mL of triethylamine was added dropwise. After the reaction solution was reacted in an ice-water mixture for 30 minutes, 18 mL of boron trifluoride diethyl ether was added dropwise, and the reaction solution was continued to react at 25° C. for 14 hours. After th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Excitation wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com