Polylactic acid dialysis membrane, preparation method thereof and dialyzer
A polylactic acid film and polylactic acid technology, applied in the field of medical equipment, can solve the problems of uneven distribution, poor blood compatibility of polylactic acid, uneven distribution of carboxylated graphene oxide, etc., and achieve good blood compatibility and good purification. effect of blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
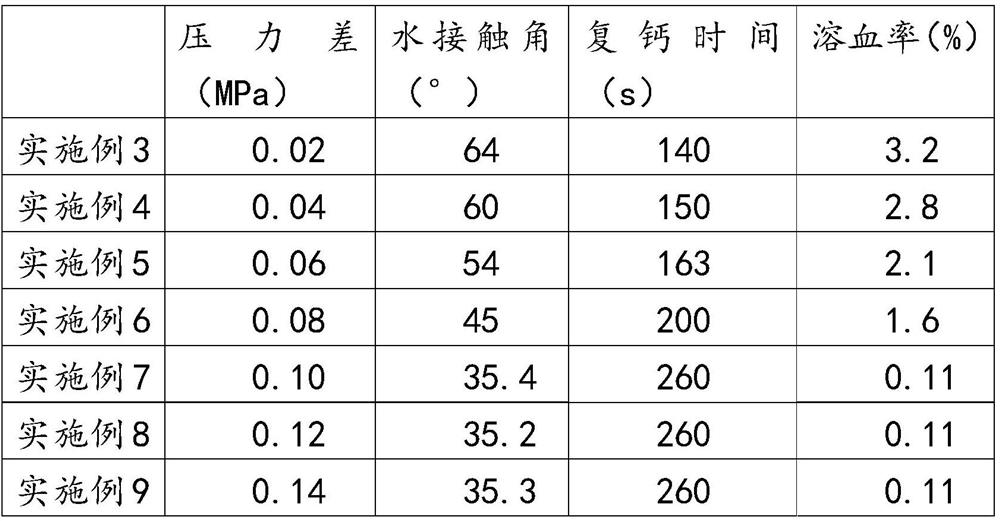
[0029] The preparation method of polylactic acid dialysis membrane is:
[0030] Before modifying the polylactic acid film, it is necessary to prepare the catechin compound solution and the carboxylated graphene oxide solution. The preparation steps of the catechin compound solution are: mix 0.1M bis(2-hydroxyethylamino) Tris(hydroxymethyl)methane is mixed with 0.6M sodium chloride to prepare a buffer solution with pH=7, and catechin compounds are dissolved in the buffer solution to prepare a buffer solution with a concentration of 0.5mg / mL~2.0mg / mL Catechin compound solution.
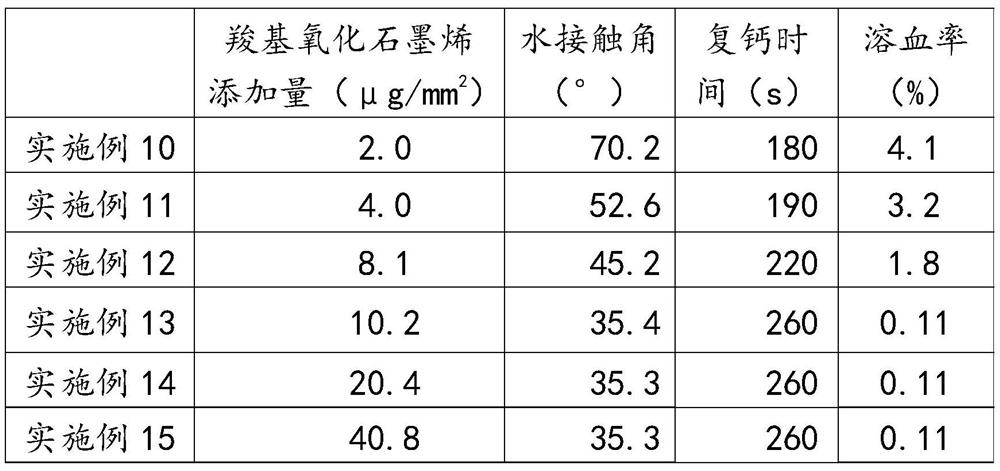
[0031] The preparation steps of carboxylated graphene oxide are: adding concentrated nitric acid and concentrated sulfuric acid to graphene oxide powder, and stirring at 60°C for 12 hours to obtain carboxylated graphene oxide; the mass-volume ratio of graphene oxide, concentrated nitric acid and concentrated sulfuric acid is 1:20:60 (m:v:v).
[0032] The modification step is as follows: soaking the poly...
Embodiment 1
[0056] Example 1 fixes carboxylated graphene oxide to the surface of a polylactic acid film by hydrogen bonding.
[0057] S1, preparation steps of catechin compound solution: mix 0.1M bis(2-hydroxyethylamino)tri(hydroxymethyl)methane with 0.6M NaCl, prepare a buffer solution with pH=7, and epicatechin Gallate was dissolved in the above buffer solution to prepare a 1 mg / mL catechin compound solution;
[0058] Preparation of carboxyl graphene oxide: Weigh 0.5g of dried graphene oxide and put it into a 100mL conical flask; first take 10mL concentrated nitric acid and add it to the conical flask, then take 30mL concentrated sulfuric acid and put it into the conical flask, add With a magnetic stirrer, keep the temperature at 60°C and stir in a water bath for 12 hours to obtain the carboxylated graphene oxide; wash the obtained carboxylated graphene oxide repeatedly with deionized water and centrifuge until the solution is neutral, then ultrasonicate the solution for 2 hours to obta...
Embodiment 2
[0072] Example 2 Carboxylated graphene oxide was immobilized on the surface of polylactic acid film through hydrogen bonding.
[0073] S1. Steps for preparing catechin compound solution: mix 0.1M bis(2-hydroxyethylamino)tris(hydroxymethyl)methane with 0.6M NaCl, prepare a buffer solution with pH=7, and dissolve tannic acid In the above buffer, prepare a 1mg / mL buffer solution;
[0074] Preparation of carboxyl graphene oxide: Weigh 0.5g of dried graphene oxide and put it into a 100mL conical flask; first take 10mL concentrated nitric acid and add it to the conical flask, then take 30mL concentrated sulfuric acid and put it into the conical flask, add With a magnetic stirrer, keep the temperature at 60°C and stir in a water bath for 12 hours to obtain the carboxylated graphene oxide; wash the obtained carboxylated graphene oxide repeatedly with deionized water and centrifuge until the solution is neutral, then ultrasonicate the solution for 2 hours to obtain carboxyl groups oxi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com