A conjugate of trehalose derivatives and carbohydrate antigens and its preparation method and application
A technology of conjugates and derivatives, applied in the field of conjugates of trehalose derivatives and sugar antigens and its preparation, can solve the problems of unstable coupling rate, complex composition, and uncertain coupling sites of glycoprotein vaccines And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
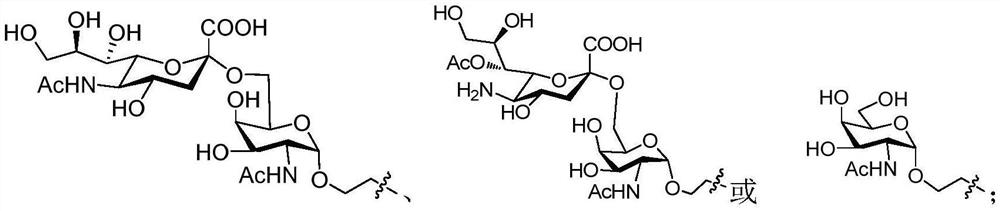
[0065] Example 1 Preparation of Trehalose Derivatives and Carbohydrate Antigen Conjugates 1, 2, 3, 4 and L2-3
[0066] 1) Synthesis of Compound 5
[0067]
[0068] Trehalose (10.00g, 0.03mol) and p-toluenesulfonic acid monohydrate (1.10g, 5.84mol) were dissolved in dimethylformamide, and anisaldehyde dimethyl acetal (20mL, 0.117 mol), stirred at room temperature for reaction, after 24 hours of reaction, diluted with ethyl acetate, saturated sodium bicarbonate solution, left standing, a white solid was precipitated, filtered by suction to obtain compound 5 (11.90 g, 70%). 1 H NMR (400MHz, CD 3 OD)δ7.41-7.39(d, J=8.8Hz, 4H), 6.88-6.68(d, J=8.8Hz, 4H), 5.51(s, 2H, PhCH), 5.11(d, J=3.6Hz, 2H,H-1&H-1′),4.19(dt,J=10.0,4.8Hz,2H,H5&H5′),4.12-4.06(m,2H,H3&H3′),4.03-3.98(t,J=9.6,2H ,H4&H4′), 3.77(s, 6H), 3.72-3.67(t, J=10.4Hz, 2H), 3.61(dd, J 1 =9.2Hz,J 2 =4.0Hz, 2H), 3.45(t, J=9.6, 2H), 3.31-3.29(m, 2H); HR-ESI-MS(m / z): calcd for C 28 h 34 o 13 [M+H] + 579.2072 found, 579.2...
Embodiment 2
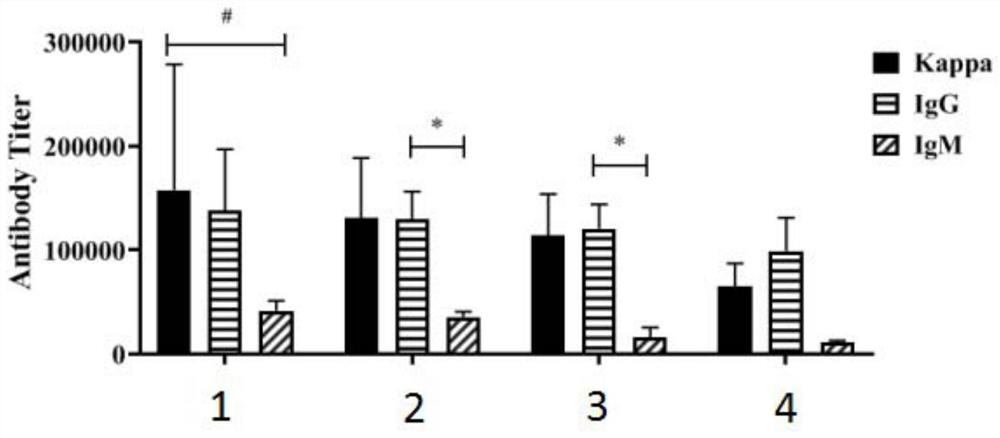
[0137] Example 2: ELISA immunoassay
[0138] (1) The prepared compounds 1 to 4 are respectively used as vaccine molecules 1, 2, 3 and 4, according to the vaccine molecule: distearoylphosphatidylcholine (DSPC): cholesterol = 1:6.5:5 dissolved in DCM -MeOH (1:1, v / v, 2mL) mixed solution, spin the solvent to form a thin layer of lipid film on the bottle wall. Add 2.0 mL of hydroxyethylpiperazine ethylsulfuric acid (HEPES) buffer solution (20 Mm, pH=7.5), and sonicate for 10-20 min to obtain liposomes 1, 2, 3 and 4.
[0139] (2) Immunization of mice: C57BL / 6 mice aged 6-8 weeks were divided into four groups, 6 mice in each group. The immunization test was carried out by subcutaneous injection in mice, and the scheme of one initial immunization and three booster immunizations was used to inject liposomes 1, 2, 3 and 4. Each injection volume is 0.1mL, and blood is collected on days 0, 27, 35, and 49. Blood is collected from each mouse at 0.1mL-0.2mL, placed at 4°C for half an hour...
Embodiment 3
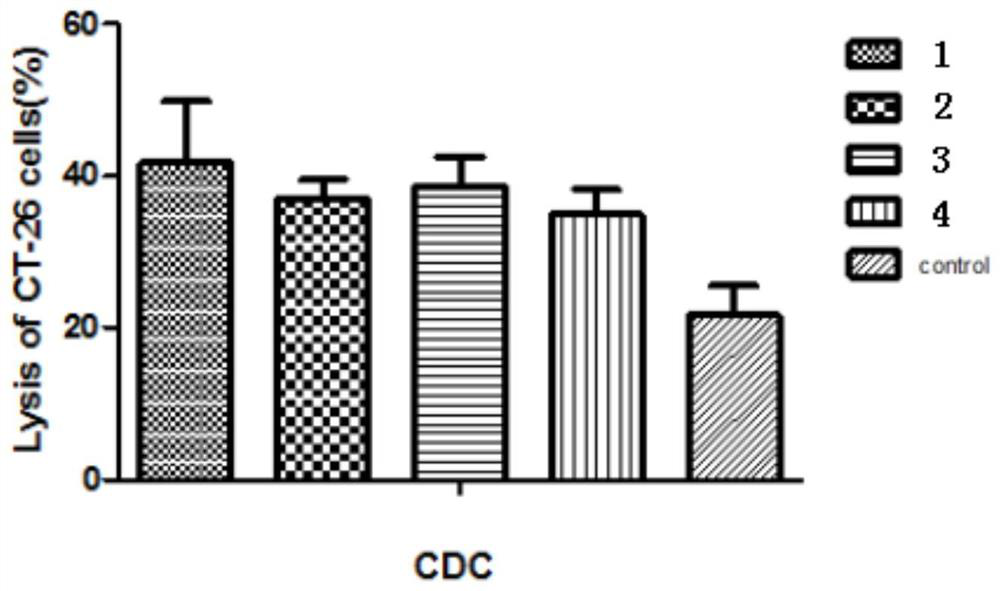
[0143] Example 3: Antibody-Mediated Complementation-Dependent Cytotoxicity (CDC)
[0144] CT-26 cells (mouse colon cancer cells) in the logarithmic growth phase were digested with trypsin, and 1 × 10 4 Cells were seeded in 96-well plates, cultured overnight at 37°C, and washed twice with serum-free 1640 medium. Take the serum samples of 6 mice in the compound 1 group that were blood drawn on day 38, take 7 μl for each sample, then mix the serum samples of the 6 mice and dilute 50 times with serum-free 1640 medium to obtain mouse serum Diluent. The mouse serum dilutions of Compound 2, Group 3, Group 4 and the blank control group were prepared according to the above method. Group 1, Group 2, Group 3, Group 4, and the blank control group (pre-immune serum) were all provided with a sample maximum enzyme activity control group, a sample control group, and a sample treatment group. Six replicate holes were made in parallel for each group. 100 μl of diluted mouse serum solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com