Enhanced MUC1 targeting chimeric antigen receptor and application thereof
A technology of chimeric antigen receptors and antigens, applied in the field of biomedicine, can solve problems such as tumor recurrence and drug resistance, and achieve the effect of improving activation activity and eliminating regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Construction of CAR Molecular Carrier
[0045] In this example, a chimeric antigen receptor αMUC1-CAR-T2 with an antigen-binding domain of an anti-MUC1 single-chain antibody and a signal transduction domain of 4-1BB, CD3ζ and TLR2 was constructed, the amino acid sequence of which is shown in SEQ ID NO: 2 Shown, the nucleic acid sequence is shown in SEQ ID NO:3;
[0046] Firstly, the coding gene of αMUC1-CAR-T2 chimeric antigen receptor (SEQ ID NO: 3) was gene-synthesized, and the restriction endonuclease Pme1 restriction site and its Protection base and restriction endonuclease Spe1 restriction site and its protection base;
[0047] The coding gene was double-digested with restriction endonucleases Pme1 and Spe1, incubated in a water bath at 37°C for 30 minutes, and the digested product containing sticky ends was recovered by agar gel electrophoresis, and ligated into a linear DNA that was also double-digested with Pme1 and Spe1. In the HopWPXLd-eGFP plasmid...
Embodiment 2
[0049] Example 2 lentiviral packaging
[0050] The lentiviral vector constructed in Example 1 was transferred into Escherichia coli, and positive single clones were selected for overnight culture, and the lentiviral vector was extracted using a plasmid extraction kit for virus packaging;
[0051] Use 293T cells for virus packaging and prepare the packaging system. The pWPXLd-expression plasmid includes a lentiviral vector containing the gene encoding αMUC1-CAR-T2 and a lentiviral vector containing the gene encoding αMUC1-CAR. The pWPXLd-eGFP plasmid does not contain CAR An empty vector encoding the gene;
[0052] The helper plasmid and the lentiviral vector with CAR molecule were simultaneously transfected into 293T cells, and the first and second viruses were harvested after 48h and 72h respectively, filtered and frozen at -80°C for use in immune cell transduction.
Embodiment 3
[0053] Example 3 T cell activation and lentiviral transfection
[0054]Mononuclear cells (PBMC) were isolated from adult peripheral blood, and total T cells were sorted out using a T cell sorting kit. After being stimulated in vitro by CD3 and CD28 antibodies for 24 hours, the overexpressed CAR molecule prepared in Example 2 was added. recombinant lentivirus;
[0055] After 12 hours of transduction, the T cells were centrifuged to change the medium; three days after the transduction, the content of GFP-positive cells was measured by flow cytometry, and the proportion of CAR-T was evaluated;
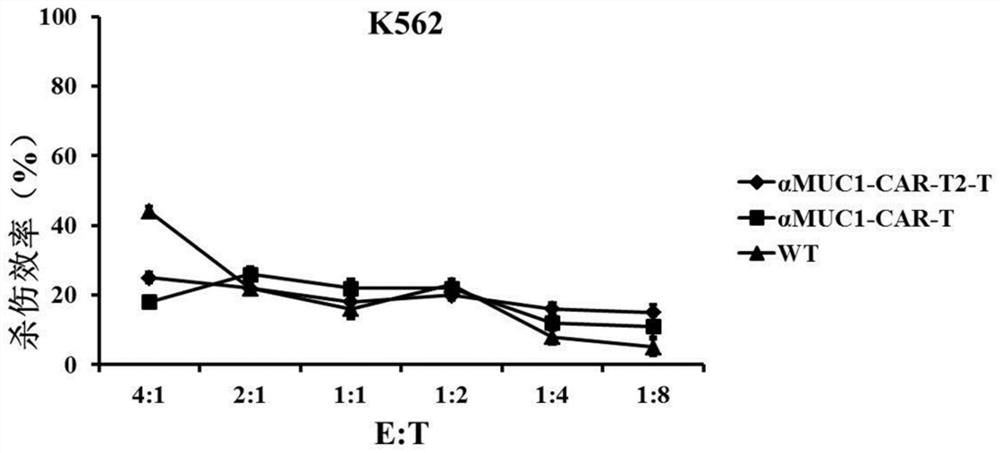
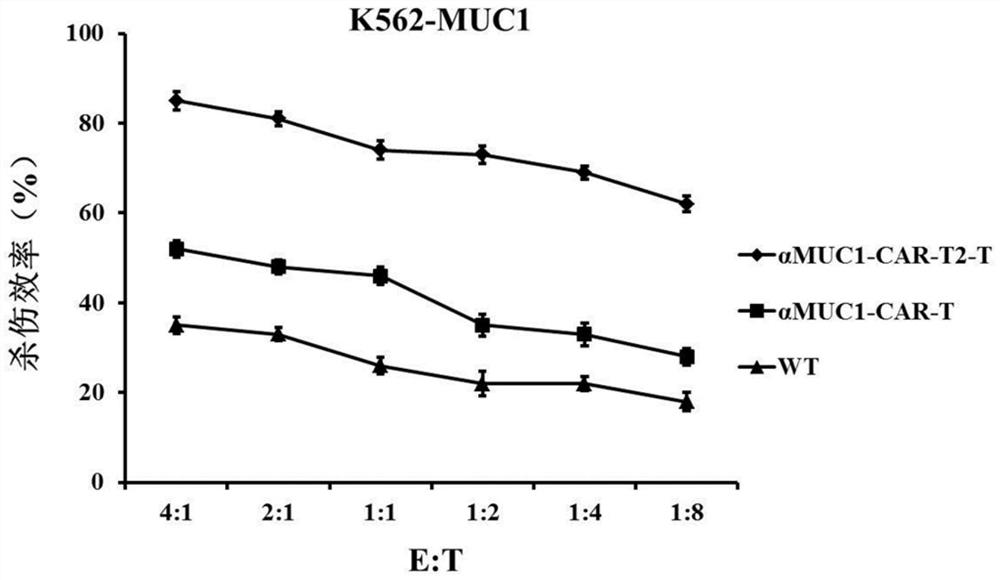
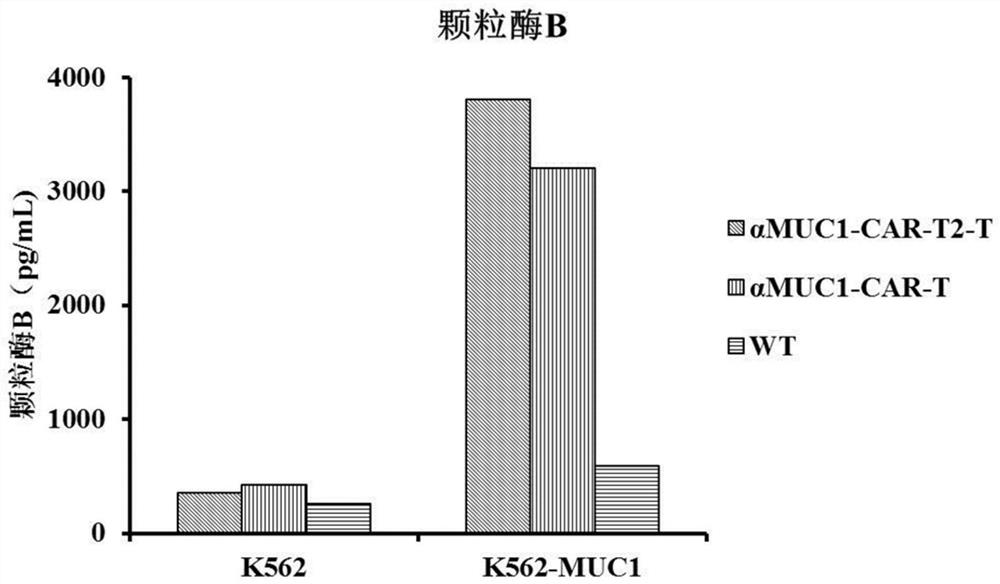
[0056] According to culture medium per milliliter 2 × 10 6 The CAR-T cells were cultured and expanded at a density of 3 cells to obtain αMUC1-CAR-T2-T, αMUC1-CAR-T and WT (transfected with blank control eGFP lentivirus) cells, and the cells were frozen ten days later until use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com