Method for preparing beta-menadione and derivative menadione sodium bisulfate of beta-menadione by taking Ce < 4 + > as oxidant
A technology of menadione and oxidant, which is applied in the preparation of quinone oxide, separation/purification of quinone, organic chemistry, etc., can solve problems such as low effect, low electrolysis efficiency, and difficulty in normal operation, and achieve good liquid-liquid mass transfer effect. , Overcome the foam problem, the effect of small solvent loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
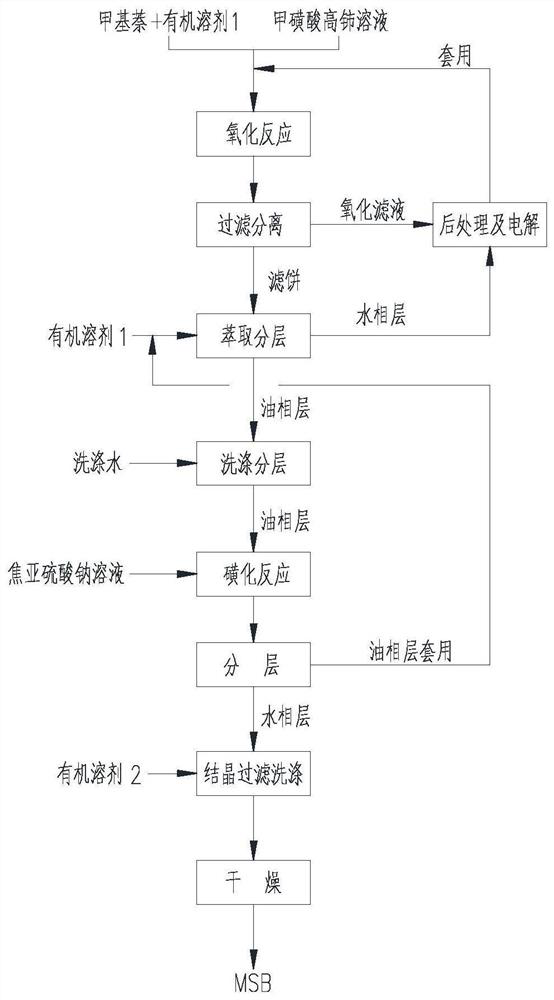
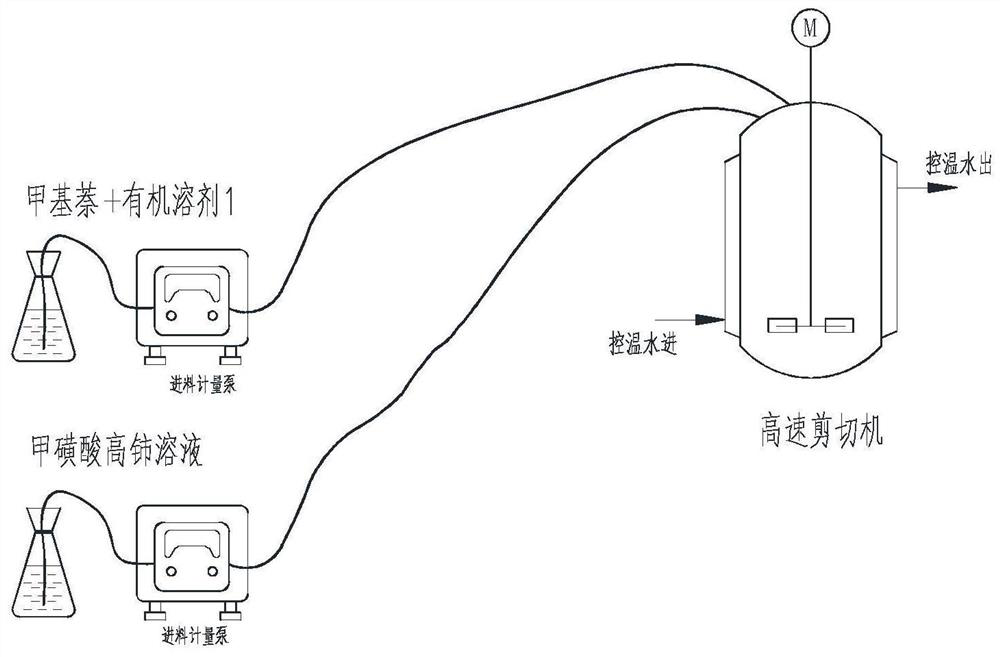
[0065] a Ce 4+ The method for preparing β-menaquinone and its derivative sodium bisulfite menadione as an oxidizing agent, the process flow diagram is as follows figure 1 As shown, the oxidation process in this method uses figure 2 The device is carried out, and the specific steps are as follows:
[0066] 1. Prepare 13.6kg of electrolyzed ceric methanesulfonate solution with a molar concentration of 0.4mol / L;
[0067] At room temperature, in a 200ml beaker, 70g of β-methylnaphthalene was put into 105g of the first organic solvent, and stirred until completely dissolved to obtain an organic solution of β-methylnaphthalene.
[0068] 2. Add 1600g of ceric methanesulfonate solution into a 20L high-speed shear emulsifier, and pass low-temperature water through the jacket to control the temperature of the material at about 10°C.
[0069] 3. Under the condition of stirring speed of 1600-2000rpm, add dropwise from two feeding ports: the remaining 12kg of ceric methanesulfonate sol...
Embodiment 2
[0079] a Ce 4+ The method for preparing β-menaquinone and its derivative sodium bisulfite menadione as an oxidizing agent, the specific steps are as follows:
[0080] 1. Prepare 13.6kg of electrolyzed ceric methanesulfonate solution with a molar concentration of 0.4mol / L;
[0081] At room temperature, in a 200ml beaker, 70g of β-methylnaphthalene was put into 105g of the first organic solvent, and stirred until completely dissolved to obtain an organic solution of β-methylnaphthalene.
[0082] 2. Add 1600g of ceric methanesulfonate solution into a 20L high-speed shear emulsifier, and pass low-temperature water through the jacket to control the temperature of the material at about 0°C.
[0083] 3. Under the condition of stirring speed of 1600-2000rpm, add dropwise from two feeding ports: the remaining 12kg of ceric methanesulfonate solution and the prepared β-methylnaphthalene organic solution, and control the dropping time for about 1.5 hours. Complete (simultaneously dropwi...
Embodiment 3
[0093] a Ce 4+ The method for preparing β-menaquinone and its derivative sodium bisulfite menadione as an oxidizing agent, the specific steps are as follows:
[0094] 1. Prepare 13.6kg of electrolyzed ceric methanesulfonate solution with a molar concentration of 0.4mol / L;
[0095]At room temperature, in a 200ml beaker, 70g of β-methylnaphthalene was put into 105g of the first organic solvent, and stirred until completely dissolved to obtain an organic solution of β-methylnaphthalene.
[0096] 2. Add 1600g of ceric methanesulfonate solution into a 20L high-speed shear emulsifier, and pass low-temperature water through the jacket to control the temperature of the material at about 10°C.
[0097] 3. Under the condition of stirring speed of 1600-2000rpm, add dropwise from two feeding ports: the remaining 12kg of ceric methanesulfonate solution and the prepared β-methylnaphthalene organic solution, and control the dropping time for about 1.5 hours. Complete (simultaneously dropwi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com